Hepatitis C virus infection among people who inject drugs in Bangkok, Thailand, 2005-2010
- PMID: 30950431
- PMCID: PMC7954142
- DOI: 10.4103/2224-3151.255350
Hepatitis C virus infection among people who inject drugs in Bangkok, Thailand, 2005-2010
Abstract
Background: Approximately 1% of adults in Thailand are infected with hepatitis C virus (HCV). New direct-acting antiviral agents achieve sustained virologic responses in >95% of HCV-infected patients and are becoming available in countries around the world. To prepare for new HCV treatment options in Thailand, this study characterized HCV infections among people who inject drugs (PWID) in Bangkok.
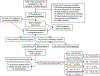
Methods: The Bangkok Tenofovir Study (BTS) was a pre-exposure prophylaxis trial conducted among PWID, 2005-2013. Blood specimens were randomly selected from PWID screened for the BTS, to test for anti-HCV antibody and HCV RNA. The HVR1 region was amplified by polymerase chain reaction, using multiplex primer sets with unique identifier sequences; amplification products were pooled in sets of 25; and consensus sequencing was performed to characterize individual HCV genotypes.
Results: The median age of 3679 participants tested for anti-HCV antibody was 31 years, 3016 (82.0%) were male and 447 (12.2%) were HIV infected. The prevalence of anti-HCV antibody was 44.3%. The adjusted odds of testing positive for anti-HCV antibody were higher in men (adjusted odds ratio [aOR] 3.2, 95% confidence interval [CI] 2.4-4.3), those aged 40 years or older (aOR 2.7, 95% CI 2.1-3.5), those who had more than a primary school education (aOR 1.7, 95% CI 1.4-2.1), and those who tested HIV positive (aOR 5.2, 95% CI 3.7-7.4). HCV RNA was detected in 644 (81.3%) of the 792 anti-HCV antibody-positive specimens, yielding an HCV RNA-positive prevalence of 36.0% (95% CI 33.8-38.2). Among a random sample of 249 of the 644 specimens, 218 could be characterized, and the most common HCV subtypes were 1a (30.3%), 1b (12.8%), 3a (35.8%), 3b (6.9%) and 6n (8.7%).
Conclusion: The prevalence of anti-HCV antibody among PWID was 44.3% and more than one third (36.0%) were HCV RNA positive. Genotypes 1, 3 and 6 accounted for all typable infections. As the government of Thailand considers introduction of direct-acting antiviral medications for people with hepatitis C, it will be important to ensure that the medications target these subtypes.
Keywords: Thailand; direct-acting antivirals; hepatitis C; people who inject drugs; viral hepatitis.
Conflict of interest statement
None
Figures
References
-
- Global hepatitis report, 2017. Geneva: World Health Organization; 2017. (http://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng..., accessed 1 November 2018).
-
- Blach S, Zeuzem S, Manns M, Altraif I, Duberg AS, Muljono DH et al.; Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76. doi:10.1016/S2468-1253(16)30181-9. - DOI - PubMed
-
- Wasitthankasem R, Vichaiwattana P, Siripon N, Posuwan N, Auphimai C, Klinfueng S et al. Liver disease burden and required treatment expenditures for hepatitis C virus (HCV) infection in Thailand: implications for HCV elimination in the new therapeutic era, a population-based study. PLoS One. 2018;13(4):e0196301. doi:10.1371/journal.pone.0196301. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
