Methyl (Alkyl)-Coenzyme M Reductases: Nickel F-430-Containing Enzymes Involved in Anaerobic Methane Formation and in Anaerobic Oxidation of Methane or of Short Chain Alkanes
- PMID: 30951290
- PMCID: PMC6941323
- DOI: 10.1021/acs.biochem.9b00164
Methyl (Alkyl)-Coenzyme M Reductases: Nickel F-430-Containing Enzymes Involved in Anaerobic Methane Formation and in Anaerobic Oxidation of Methane or of Short Chain Alkanes
Abstract
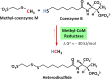
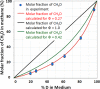
Methyl-coenzyme M reductase (MCR) catalyzes the methane-forming step in methanogenic archaea. The active enzyme harbors the nickel(I) hydrocorphin coenzyme F-430 as a prosthetic group and catalyzes the reversible reduction of methyl-coenzyme M (CH3-S-CoM) with coenzyme B (HS-CoM) to methane and CoM-S-S-CoB. MCR is also involved in anaerobic methane oxidation in reverse of methanogenesis and most probably in the anaerobic oxidation of ethane, propane, and butane. The challenging question is how the unreactive CH3-S thioether bond in methyl-coenzyme M and the even more unreactive C-H bond in methane and the other hydrocarbons are anaerobically cleaved. A key to the answer is the negative redox potential (Eo') of the Ni(II)F-430/Ni(I)F-430 couple below -600 mV and the radical nature of Ni(I)F-430. However, the negative one-electron redox potential is also the Achilles heel of MCR; it makes the nickel enzyme one of the most O2-sensitive enzymes known to date. Even under physiological conditions, the Ni(I) in MCR is oxidized to the Ni(II) or Ni(III) states, e.g., when in the cells the redox potential (E') of the CoM-S-S-CoB/HS-CoM and HS-CoB couple (Eo' = -140 mV) gets too high. Methanogens therefore harbor an enzyme system for the reactivation of inactivated MCR in an ATP-dependent reduction reaction. Purification of active MCR in the Ni(I) oxidation state is very challenging and has been achieved in only a few laboratories. This perspective reviews the function, structure, and properties of MCR, what is known and not known about the catalytic mechanism, how the inactive enzyme is reactivated, and what remains to be discovered.
Conflict of interest statement
The author declares no competing financial interest.
Figures









References
-
- Taylor C. D.; Wolfe R. S. (1974) Structure and methylation of coenzyme M (HSCH2CH2SO3). J. Biol. Chem. 249, 4879–4885. - PubMed
-
- Gunsalus R. P.; Wolfe R. S. (1976) Components and cofactors for enzymatic formation of methane from methyl-coenzyme-M. Fed Proc. 35, 1547–1547.
-
- Gunsalus R. P.; Wolfe R. S. (1980) Methyl coenzyme-M reductase from Methanobacterium thermoautotrophicum - Resolution and properties of the components. J. Biol. Chem. 255, 1891–1895. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

