Regulation of Transmembrane Signaling by Phase Separation
- PMID: 30951647
- PMCID: PMC6771929
- DOI: 10.1146/annurev-biophys-052118-115534
Regulation of Transmembrane Signaling by Phase Separation
Abstract
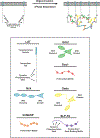
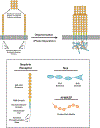
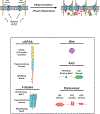
Cell surface transmembrane receptors often form nanometer- to micrometer-scale clusters to initiate signal transduction in response to environmental cues. Extracellular ligand oligomerization, domain-domain interactions, and binding to multivalent proteins all contribute to cluster formation. Here we review the current understanding of mechanisms driving cluster formation in a series of representative receptor systems: glycosylated receptors, immune receptors, cell adhesion receptors, Wnt receptors, and receptor tyrosine kinases. We suggest that these clusters share properties of systems that undergo liquid-liquid phase separation and could be investigated in this light.
Keywords: biomolecular condensates; cell signaling; phase separation; receptor clusters; receptor organization.
Figures




References
-
- Ahmad N, Gabius H-J, André S, Kaltner H, Sabesan S, et al. 2004. Galectin-3 Precipitates as a Pentamer with Synthetic Multivalent Carbohydrates and Forms Heterogeneous Cross-linked Complexes. J. Biol. Chem. 279(12):10841–47 - PubMed
-
- Asherie N, Pande J, Lomakin A, Ogun O, Hanson SRA, et al. 1998. Oligomerization and phase separation in globular protein solutions. Biophys. Chem. 75(3):213–27 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

