Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory
- PMID: 30951669
- PMCID: PMC6499401
- DOI: 10.1016/j.cell.2019.02.016
Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory
Abstract
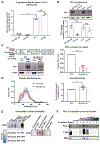
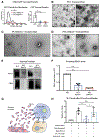
PD-L1 on the surface of tumor cells binds its receptor PD-1 on effector T cells, thereby suppressing their activity. Antibody blockade of PD-L1 can activate an anti-tumor immune response leading to durable remissions in a subset of cancer patients. Here, we describe an alternative mechanism of PD-L1 activity involving its secretion in tumor-derived exosomes. Removal of exosomal PD-L1 inhibits tumor growth, even in models resistant to anti-PD-L1 antibodies. Exosomal PD-L1 from the tumor suppresses T cell activation in the draining lymph node. Systemically introduced exosomal PD-L1 rescues growth of tumors unable to secrete their own. Exposure to exosomal PD-L1-deficient tumor cells suppresses growth of wild-type tumor cells injected at a distant site, simultaneously or months later. Anti-PD-L1 antibodies work additively, not redundantly, with exosomal PD-L1 blockade to suppress tumor growth. Together, these findings show that exosomal PD-L1 represents an unexplored therapeutic target, which could overcome resistance to current antibody approaches.
Keywords: CD274; NSMASE2; PD-L1; RAB27A; SMPD3; T cells; abscopal; cancer; exosomes; immune checkpoint.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





References
-
- Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, and Min WP (2005). Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis 35, 169–173. - PubMed
-
- Carr B, and Warren J (2012). Company profile: NanoSight: delivering practical solutions for biological nanotechnology. Nanomedicine (Lond.) 7, 1129–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

