Targeting High Mobility Group Box-1 (HMGB1) Promotes Cell Death in Myelodysplastic Syndrome
- PMID: 30952643
- PMCID: PMC6800136
- DOI: 10.1158/1078-0432.CCR-18-3517
Targeting High Mobility Group Box-1 (HMGB1) Promotes Cell Death in Myelodysplastic Syndrome
Abstract
Purpose: Myelodysplastic syndrome (MDS) is associated with a dysregulated innate immune system. The purpose of this study was to determine whether modulation of the innate immune system via high mobility group box-1 (HMGB1) could reduce cell viability in MDS.
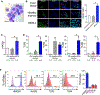
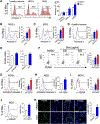
Experimental design: We quantified HMGB1 in an MDS cell line MDS-L and in primary MDS cells compared with nonmalignant hematopoietic cells. We performed loss-of-function studies of HMGB1 using pooled siRNAs and a small-molecule inhibitor sivelestat compared with standard chemotherapy. We measured levels of engraftment of MDS-L cells in NOD-scidIL2Rgnull (NSG) mice following treatment with sivelestat. Mechanistically, we interrogated cell survival pathways and 45 targets within the NFκB pathway using both protein analysis and a proteome profiler array.
Results: We discovered that HMGB1 had increased expression in both MDS-L cells and in primary CD34+ MDS cells compared with healthy CD34+ hematopoietic cells. Sivelestat impaired MDS cell expansion, increased cellular death, and spared healthy hematopoietic cells. MDS-L marrow engraftment is reduced significantly at 17 weeks following treatment with sivelestat compared with control mice. Treatment of CD34+ MDS cells with sivelestat and azacitidine or decitabine was additive to increase apoptotic cell death compared with chemotherapy alone. Sivelestat promoted apoptosis with increased expression of PUMA, activated caspase 3, and increased DNA double-strand breaks. Inhibition of HMGB1 reduced levels of Toll-like receptors (TLR) and suppressed activation of NFκB in MDS-L cells.
Conclusions: Inhibition of HMGB1 could promote MDS cell death and alter innate immune responses via suppression of NFκB pathways.
©2019 American Association for Cancer Research.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures




References
-
- Sekeres MA, and Cutler C. How we treat higher-risk myelodysplastic syndromes. Blood 2014;123:829–36. - PubMed
-
- Mukherjee S, Boccaccio D, Sekeres MA, and Copelan E. Allogeneic hematopoietic cell transplantation for myelodysplastic syndromes: Lingering uncertainties and emerging possibilities. Biol Blood Marrow Transplant 2015;21:412–20. - PubMed
-
- Maratheftis Cl AE, Moutsopoulos HM, et al. Toll-like receptor-4 is upregulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clin Cancer Res 2007;13:1154–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

