INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease
- PMID: 30952794
- PMCID: PMC6512884
- DOI: 10.1212/WNL.0000000000007232
INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease
Erratum in
-
INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease.Neurology. 2019 Aug 20;93(8):371. doi: 10.1212/WNL.0000000000007919. Neurology. 2019. PMID: 31427496 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the safety and efficacy of low-dose naproxen for prevention of progression in presymptomatic Alzheimer disease (AD) among cognitively intact persons at risk.
Methods: Investigation of Naproxen Treatment Effects in Pre-symptomatic Alzheimer's Disease (INTREPAD), a 2-year double-masked pharmaco-prevention trial, enrolled 195 AD family history-positive elderly (mean age 63 years) participants screened carefully to exclude cognitive disorder (NCT-02702817). These were randomized 1:1 to naproxen sodium 220 mg twice daily or placebo. Multimodal imaging, neurosensory, cognitive, and (in ∼50%) CSF biomarker evaluations were performed at baseline, 3, 12, and 24 months. A modified intent-to-treat analysis considered 160 participants who remained on-treatment through their first follow-up examination. The primary outcome was rate of change in a multimodal composite presymptomatic Alzheimer Progression Score (APS).
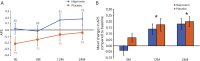
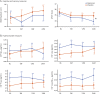
Results: Naproxen-treated individuals showed a clear excess of adverse events. Among treatment groups combined, the APS increased by 0.102 points/year (SE 0.014; p < 10-12), but rate of change showed little difference by treatment assignment (0.019 points/year). The treatment-related rate ratio of 1.16 (95% confidence interval 0.64-1.96) suggested that naproxen does not reduce the rate of APS progression by more than 36%. Secondary analyses revealed no notable treatment effects on individual CSF, cognitive, or neurosensory biomarker indicators of progressive presymptomatic AD.
Conclusions: In cognitively intact individuals at risk, sustained treatment with naproxen sodium 220 mg twice daily increases frequency of adverse health effects but does not reduce apparent progression of presymptomatic AD.
Classification of evidence: This study provides Class I evidence that, for people who are cognitively intact, low-dose naproxen does not significantly reduce progression of a composite indicator of presymptomatic AD.
Copyright © 2019 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


Comment in
-
Naproxen for presymptomatic Alzheimer disease: Is this the end, or shall we try again?Neurology. 2019 Apr 30;92(18):829-830. doi: 10.1212/WNL.0000000000007233. Epub 2019 Apr 5. Neurology. 2019. PMID: 30952790 No abstract available.
-
Can naproxen slow the progression of Alzheimer disease?Neurology. 2019 Apr 30;92(18):e2181-e2184. doi: 10.1212/WNL.0000000000007418. Neurology. 2019. PMID: 31036584 No abstract available.
-
Reader response: INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease.Neurology. 2020 Mar 31;94(13):593-594. doi: 10.1212/WNL.0000000000009184. Neurology. 2020. PMID: 32229635 No abstract available.
-
Author response: INTREPAD: A randomized trial of naproxen to slow progress of presymptomatic Alzheimer disease.Neurology. 2020 Mar 31;94(13):594. doi: 10.1212/WNL.0000000000009185. Neurology. 2020. PMID: 32229636 No abstract available.
References
-
- in t' Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N Engl J Med 2001;345:1515–1521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
