Pantoprazole Affecting Docetaxel Resistance Pathways via Autophagy (PANDORA): Phase II Trial of High Dose Pantoprazole (Autophagy Inhibitor) with Docetaxel in Metastatic Castration-Resistant Prostate Cancer (mCRPC)
- PMID: 30952818
- PMCID: PMC6738292
- DOI: 10.1634/theoncologist.2018-0621
Pantoprazole Affecting Docetaxel Resistance Pathways via Autophagy (PANDORA): Phase II Trial of High Dose Pantoprazole (Autophagy Inhibitor) with Docetaxel in Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Abstract
Background: Enhancing the effectiveness of docetaxel for men with metastatic castration-resistant prostate cancer (mCRPC) is an unmet clinical need. Preclinical studies demonstrated that high-dose pantoprazole can prevent or delay resistance to docetaxel via the inhibition of autophagy in several solid tumor xenografts.
Materials and methods: Men with chemotherapy-naive mCRPC with a prostate-specific antigen (PSA) >10 ng/mL were eligible for enrolment. Men received intravenous pantoprazole (240 mg) prior to docetaxel (75 mg/m2) every 21 days, with continuous prednisone 5 mg twice daily. Primary endpoint was a confirmed ≥50% decline of PSA. The trial used a Simon's two-stage design.
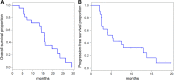
Results: Between November 2012 and March 2015, 21 men with a median age of 70 years (range, 58-81) were treated (median, 6 cycles; range, 2-11). Men had received prior systemic therapies (median, 1; range, 0-3), and 14 had received abiraterone and/or enzalutamide. PSA response rate was 52% (11/21), which did not meet the prespecified criterion (≥13/21 responders) to proceed to stage 2 of the study. At interim analysis with a median follow-up of 17 months, 18 (86%) men were deceased (15 castration-resistant prostate cancer, 2 unknown, 1 radiation complication). Of the men with RECIST measurable disease, the radiographic partial response rate was 31% (4/13). The estimated median overall survival was 15.7 months (95% confidence interval [CI], 9.3-19.6) and median PFS was 5.3 months (95% CI, 2.6-12.9). There were no toxic deaths, and all adverse events were attributed to docetaxel.
Conclusion: The combination of docetaxel and pantoprazole was tolerable, but the resultant clinical activity was not sufficient to meet the ambitious predefined target to warrant further testing.
Implications for practice: To date, no docetaxel combination regimen has reported superior efficacy over docetaxel alone in men with metastatic castration-resistant prostate cancer (mCRPC). The PANDORA trial has demonstrated that the combination of high dose pantoprazole with docetaxel is tolerable, but the clinical activity was not sufficient to warrant further testing. The chemotherapy standard of care for men with mCRPC remains docetaxel with prednisone. Future studies of autophagy inhibitors will need to measure autophagy inhibition accurately and determine the degree of autophagy inhibition required to produce a meaningful clinical response.
Keywords: Autophagy inhibition; Metastatic castration‐resistant prostate cancer.
© AlphaMed Press 2019.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
References
-
- Karpathiou G, Sivridis E, Koukourakis MI et al. Light‐chain 3A autophagic activity and prognostic significance in non‐small cell lung carcinomas. Chest 2011;140:127–134. - PubMed
-
- Sivridis E, Koukourakis MI, Mendrinos SE et al. Beclin‐1 and LC3A expression in cutaneous malignant melanomas: A biphasic survival pattern for beclin‐1. Melanoma Res 2011;21:188–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous