Perfluorocarbon regulates the intratumoural environment to enhance hypoxia-based agent efficacy
- PMID: 30952842
- PMCID: PMC6450981
- DOI: 10.1038/s41467-019-09389-2
Perfluorocarbon regulates the intratumoural environment to enhance hypoxia-based agent efficacy
Abstract
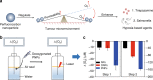
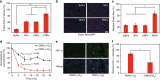
Hypoxia-based agents (HBAs), such as anaerobic bacteria and bioreductive prodrugs, require both a permeable and hypoxic intratumoural environment to be fully effective. To solve this problem, herein, we report that perfluorocarbon nanoparticles (PNPs) can be used to create a long-lasting, penetrable and hypoxic tumour microenvironment for ensuring both the delivery and activation of subsequently administered HBAs. In addition to the increased permeability and enhanced hypoxia caused by the PNPs, the PNPs can be retained to further achieve the long-term inhibition of intratumoural O2 reperfusion while enhancing HBA accumulation for over 24 h. Therefore, perfluorocarbon materials may have great potential for reigniting clinical research on hypoxia-based drugs.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

