Roxadustat Treatment of Chronic Kidney Disease-Associated Anemia in Japanese Patients Not on Dialysis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 30953333
- PMCID: PMC6824366
- DOI: 10.1007/s12325-019-00943-4
Roxadustat Treatment of Chronic Kidney Disease-Associated Anemia in Japanese Patients Not on Dialysis: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Introduction: This study evaluated efficacy and safety/tolerability of roxadustat, an oral hypoxia-inducible factor prolyl hydroxylase inhibitor, in Japanese anemic non-dialysis-dependent chronic kidney disease (NDD-CKD) patients.
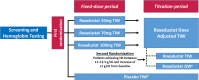
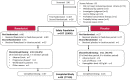
Methods: In this phase 2, double-blind, 24-week study, NDD-CKD patients were randomized to oral placebo or roxadustat (50, 70, or 100 mg) three times weekly (TIW) for 6 weeks followed by dose adjustments to maintain hemoglobin (Hb) at 10-12 g/dL for 18 weeks; patients meeting pre-defined criteria were re-randomized to TIW or once-weekly dosing. The primary end point was rate of rise of Hb (g/dL/week) during the first 6 weeks; secondary end points included response rate (Hb ≥ 10.0 g/dL and increase in Hb from baseline ≥ 1 g/dL) and mean Hb and change from baseline in Hb at weeks 18-24. The main safety outcomes were vital signs, laboratory test results, electrocardiograms, and frequency of treatment-emergent adverse events.
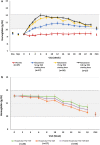
Results: Of 107 patients randomized, 83 completed the study. The mean (SD) rate of rise of Hb during the first 6 weeks was - 0.052 (0.142) for placebo and + 0.200 (0.160), + 0.453 (0.256), and + 0.570 (0.240) for roxadustat 50-, 70-, and 100-mg TIW groups, respectively (p < 0.001). Response rate was 14.8% for placebo and 81.5%, 100%, and 100% for roxadustat TIW groups (p < 0.001). Change in Hb from baseline at weeks 18-24 was - 0.17 (0.61) for placebo and + 1.10 (0.71), + 1.33 (0.82), and + 1.55 (0.88) g/dL for roxadustat TIW groups (p < 0.001). No deaths or major adverse cardiac events occurred with roxadustat.
Conclusion: Roxadustat was well tolerated and effective in correcting Hb levels within 6 weeks in Japanese anemic NDD-CKD patients.
Trial registration: ClinicalTrials.gov: NCT01964196. Registered 15 October 2013 (retrospectively registered).
Funding: Astellas Pharma Inc.
Keywords: Anemia; CKD; Clinical trial; Hemoglobin; Nephrology; Roxadustat.
Figures

References
-
- KDOQI National Kidney Foundation KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006;47(5 Suppl 3):S11–S145. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279–335.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

