72-Hour in vivo evaluation of nitric oxide generating artificial lung gas exchange fibers in sheep
- PMID: 30953800
- PMCID: PMC6513705
- DOI: 10.1016/j.actbio.2019.04.004
72-Hour in vivo evaluation of nitric oxide generating artificial lung gas exchange fibers in sheep
Abstract
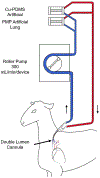
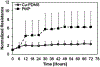
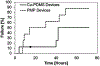
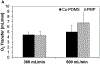
The large, densely packed artificial surface area of artificial lungs results in rapid clotting and device failure. Surface generated nitric oxide (NO) can be used to reduce platelet activation and coagulation on gas exchange fibers, while not inducing patient bleeding due to its short half-life in blood. To generate NO, artificial lungs can be manufactured with PDMS hollow fibers embedded with copper nanoparticles (Cu NP) and supplied with an infusion of the NO donor S-nitroso-N-acetyl-penicillamine (SNAP). The SNAP reacts with Cu NP to generate NO. This study investigates clot formation and gas exchange performance of artificial lungs with either NO-generating Cu-PDMS or standard polymethylpentene (PMP) fibers. One miniature artificial lung (MAL) made with 10 wt% Cu-PDMS hollow fibers and one PMP control MAL were attached to sheep in parallel in a veno-venous extracorporeal membrane oxygenation circuit (n = 8). Blood flow through each device was set at 300 mL/min, and each device received a SNAP infusion of 0.12 μmol/min. The ACT was between 110 and 180 s in all cases. Blood flow resistance was calculated as a measure of clot formation on the fiber bundle. Gas exchange experiments comparing the two groups were conducted every 24 h at blood flow rates of 300 and 600 mL/min. Devices were removed once the resistance reached 3x baseline (failure) or following 72 h. All devices were imaged using scanning electron microscopy (SEM) at the inlet, outlet, and middle of the fiber bundle. The Cu-PDMS NO generating MALs had a significantly smaller increase in resistance compared to the control devices. Resistance rose from 26 ± 8 and 23 ± 5 in the control and Cu-PDMS devices, respectively, to 35 ± 8 mmHg/(mL/min) and 72 ± 23 mmHg/(mL/min) at the end of each experiment. The resistance and SEM imaging of fiber surfaces demonstrate lower clot formation on Cu-PDMS fibers. Although not statistically significant, oxygen transfer for the Cu-PDMS MALs was 13.3% less than the control at 600 mL/min blood flow rate. Future in vivo studies with larger Cu-PDMS devices are needed to define gas exchange capabilities and anticoagulant activity over a long-term study at clinically relevant ACTs. STATEMENT OF SIGNIFICANCE: In artificial lungs, the large, densely-packed blood contacting surface area of the hollow fiber bundle is critical for gas exchange but also creates rapid, surface-generated clot requiring significant anticoagulation. Monitoring of anticoagulation, thrombosis, and resultant complications has kept permanent respiratory support from becoming a clinical reality. In this study, we use a hollow fiber material that generates nitric oxide (NO) to prevent platelet activation at the blood contacting surface. This material is tested in vivo in a miniature artificial lung and compared against the clinical standard. Results indicated significantly reduced clot formation. Surface-focused anticoagulation like this should reduce complication rates and allow for permanent respiratory support by extending the functional lifespan of artificial lungs and can further be applied to other medical devices.
Keywords: Anti-platelet; Artificial lung; Coagulation; Nitric oxide; Oxygenator.
Copyright © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure
There are zero conflicts of interest between the authors and this study.
Figures
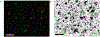



References
-
- Fischer S, Simon AR, Welte T, Hoeper MM, Meyer A, Tessmann R, Gohrbandt B, Gottlieb J, Haverich A, Strueber M, Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung, J. Thorac. Cardiovasc. Surg 131 (2006) 719–723. doi:10.1016/j.jtcvs.2005.10.050. - DOI - PubMed
-
- Strueber M, Hoeper MM, Fischer S, Cypel M, Warnecke G, Gottlieb J, Pierre A, Welte T, Haverich A, Simon AR, Keshavjee S, Bridge to thoracic organ transplantation in patients with pulmonary arterial hypertension using a pumpless lung assist device, Am. J. Transplant 9 (2009) 853–857. doi:10.1111/j.1600-6143.2009.02549.x. - DOI - PubMed
-
- Maul TM, Massicotte PM, Wearden PD, ECMO Biocompatibility: Surface coatings, anticoagulation, and coagulation monitoring, in: Extracorpor. Membr. Oxyg. - Adv. Ther, 2016: pp. 27–56.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

