Cell type and circuit modules in the spinal cord
- PMID: 30954861
- PMCID: PMC8559966
- DOI: 10.1016/j.conb.2019.03.003
Cell type and circuit modules in the spinal cord
Abstract
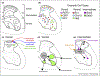
The spinal cord contains an extraordinarily diverse population of interconnected neurons to process somatosensory information and execute movement. Studies of the embryonic spinal cord have elucidated basic principles underlying the specification of spinal cord neurons, while adult and postnatal studies have provided insight into cell type function and circuitry. However, the overarching principles that bridge molecularly defined subtypes with their connectivity, physiology, and function remain unclear. This review consolidates recent work in spinal neuron characterization, examining how molecular and spatial features of individual spinal neuron types relate to the reference points of connectivity and function. This review will focus on how spinal neuron subtypes are organized to control movement in the mouse.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest statement
Nothing declared.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

