The therapeutic challenge of late antibody-mediated kidney allograft rejection
- PMID: 30955215
- PMCID: PMC6850109
- DOI: 10.1111/tri.13436
The therapeutic challenge of late antibody-mediated kidney allograft rejection
Abstract
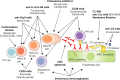
Late antibody-mediated rejection (ABMR) is a cardinal cause of kidney allograft failure, manifesting as a continuous and, in contrast with early rejection, often clinically silent alloimmune process. While significant progress has been made towards an improved understanding of its molecular mechanisms and the definition of diagnostic criteria, there is still no approved effective treatment. In recent small randomized controlled trials, therapeutic strategies with promising results in observational studies, such as proteasome inhibitor bortezomib, anti-C5 antibody eculizumab, or high dose intravenous immunoglobulin plus rituximab, had no significant impact in late and/or chronic ABMR. Such disappointing results reinforce a need of new innovative treatment strategies. Potential candidates may be the interference with interleukin-6 to modulate B cell alloimmunity, or innovative compounds that specifically target antibody-producing plasma cells, such as antibodies against CD38. Given the phenotypic heterogeneity of ABMR, the design of adequate systematic trials to assess the safety and efficiency of such therapies, however, is challenging. Several trials are currently being conducted, and new developments will hopefully provide us with effective ways to counteract the deleterious impact of antibody-mediated graft injury. Meanwhile, the weight of evidence would suggest that, when approaching using existing treatments for established antibody-mediated rejection, "less may be more".
Keywords: antibody-mediated rejection; kidney transplantation; randomized controlled trial; rejection treatment.
© 2019 The Authors. Transplant International published by John Wiley & Sons Ltd on behalf of Steunstichting ESOT.
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures
References
-
- Lefaucheur C, Loupy A. Antibody‐mediated rejection of solid‐organ allografts. N Engl J Med 2018; 379: 2580. - PubMed
-
- Lachmann N, Niemann M, Reinke P, et al Donor‐recipient matching based on predicted indirectly recognizable HLA epitopes independently predicts the incidence of de novo donor‐specific HLA antibodies following renal transplantation. Am J Transplant 2017; 17: 3076. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

