Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity
- PMID: 30955881
- PMCID: PMC6954108
- DOI: 10.1016/j.cell.2019.02.005
Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity
Abstract
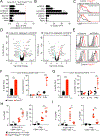
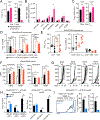
Differentiation of proinflammatory CD4+ conventional T cells (Tconv) is critical for productive antitumor responses yet their elicitation remains poorly understood. We comprehensively characterized myeloid cells in tumor draining lymph nodes (tdLN) of mice and identified two subsets of conventional type-2 dendritic cells (cDC2) that traffic from tumor to tdLN and present tumor-derived antigens to CD4+ Tconv, but then fail to support antitumor CD4+ Tconv differentiation. Regulatory T cell (Treg) depletion enhanced their capacity to elicit strong CD4+ Tconv responses and ensuing antitumor protection. Analogous cDC2 populations were identified in patients, and as in mice, their abundance relative to Treg predicts protective ICOS+ PD-1lo CD4+ Tconv phenotypes and survival. Further, in melanoma patients with low Treg abundance, intratumoral cDC2 density alone correlates with abundant CD4+ Tconv and with responsiveness to anti-PD-1 therapy. Together, this highlights a pathway that restrains cDC2 and whose reversal enhances CD4+ Tconv abundance and controls tumor growth.
Keywords: CD4(+) T cells; T cell priming; checkpoint blockade; dendritic cells; immunotherapy; regulatory T cells; tumor immunology; tumor microenvironment.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures





References
-
- Alferink J, Lieberam I, Reindl W, Behrens A, Weiss S, Huser N, Gerauer K, Ross R, Reske-Kunz AB, Ahmad-Nejad P, et al. (2003). Compartmentalized production of CCL17 in vivo: strong inducibility in peripheral dendritic cells contrasts selective absence from the spleen. The Journal of experimental medicine 197, 585–599. - PMC - PubMed
-
- Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J, Verthuy C, Davanture S, Azukizawa H, Flores-Langarica A, et al. (2015). Homeostatic NF-kappaB Signaling in Steady-State Migratory Dendritic Cells Regulates Immune Homeostasis and Tolerance. Immunity 42, 627–639. - PubMed
-
- Baratin M, Simon L, Jorquera A, Ghigo C, Dembele D, Nowak J, Gentek R, Wienert S, Klauschen F, Malissen B, et al. (2017). T Cell Zone Resident Macrophages Silently Dispose of Apoptotic Cells in the Lymph Node. Immunity 47, 349–362.e345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

