Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage
- PMID: 30955887
- PMCID: PMC6474841
- DOI: 10.1016/j.cell.2019.02.028
Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage
Abstract
Neutrophils are attracted to and generate dense swarms at sites of cell damage in diverse tissues, often extending the local disruption of organ architecture produced by the initial insult. Whether the inflammatory damage resulting from such neutrophil accumulation is an inescapable consequence of parenchymal cell death has not been explored. Using a combination of dynamic intravital imaging and confocal multiplex microscopy, we report here that tissue-resident macrophages rapidly sense the death of individual cells and extend membrane processes that sequester the damage, a process that prevents initiation of the feedforward chemoattractant signaling cascade that results in neutrophil swarms. Through this "cloaking" mechanism, the resident macrophages prevent neutrophil-mediated inflammatory damage, maintaining tissue homeostasis in the face of local cell injury that occurs on a regular basis in many organs because of mechanical and other stresses. VIDEO ABSTRACT.
Keywords: anti-inflammation; cloaking; collateral damage; damage response; inflammation prevention; intravital imaging; neutrophil swarming; resident macrophages; tissue homeostasis; tissue protection.
Published by Elsevier Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures
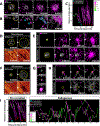
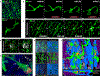
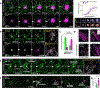
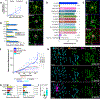
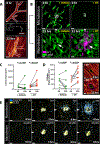
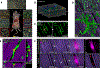
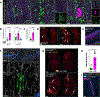
Comment in
-
"Cloaking" on Time: A Cover-Up Act by Resident Tissue Macrophages.Cell. 2019 Apr 18;177(3):514-516. doi: 10.1016/j.cell.2019.03.042. Cell. 2019. PMID: 31002790
-
Damage cloaked by macs.Nat Rev Immunol. 2019 Jun;19(6):352-353. doi: 10.1038/s41577-019-0170-3. Nat Rev Immunol. 2019. PMID: 31019285 No abstract available.
References
-
- Amit I, Winter DR, and Jung S (2016). The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 17, 18–25. - PubMed
-
- Cekic C, and Linden J (2016). Purinergic regulation of the immune system. Nat Rev Immunol 16, 177–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

