An optimized assay for detecting Encephalitozoon intestinalis and Enterocytozoon bieneusi in dairy calf feces using polymerase chain reaction technology
- PMID: 30956449
- PMCID: PMC6423185
- DOI: 10.1007/s12639-018-1060-5
An optimized assay for detecting Encephalitozoon intestinalis and Enterocytozoon bieneusi in dairy calf feces using polymerase chain reaction technology
Abstract
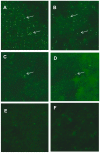
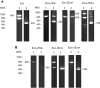
The purpose of this study was to optimize primary and nested polymerase chain reaction (PCR) assays for detecting the microsporidia Encephalitozoon intestinalis and Enterocytozoon bieneusi in fecal samples from dairy calves. PCR for these microsporidia were compared to immunofluorescence assays (IFA) based on commercially available monoclonal antibodies specific for outer wall proteins of Enc. intestinalis or Ent. bieneusi. Fecal samples were collected from 15 dairy calves and processed by molecular sieving followed by salt floatation to recover Enc. intestinalis and Ent. bieneusi spores. An aliquot of the final supernatant was applied to glass slides for IFA testing; another aliquot was extracted for total DNA using a QIAamp Stool Mini-Kit for primary and nested Enc. intestinalis- and Ent. bieneusi-specific PCR analysis. Internal standards were generated for both Enc. intestinalis and Ent. bieneusi PCR assays to control for false negative reactions due to the presence of inhibitors commonly found in fecal samples. Using the commercial MicrosporIFA (Waterborne, Inc.) as the gold standard, the optimized Enc. intestinalis PCR method provided 85.7% sensitivity and 100% specificity with a kappa value = 0.865. Likewise, using the commercial BienusiGlo IFA (Waterborne, Inc.) as the gold standard, the optimized Ent. bieneusi PCR method provided 83.3% sensitivity and 100% specificity with a kappa value = 0.857. Sequencing of amplicons from both PCR assays confirmed the presence of Enc. intestinalis or Ent. bieneusi. In conclusion, our optimized assays for recovering and detecting Enc. intestinalis or Ent. bieneusi in feces from dairy calves provides a valuable alternative to traditional IFA methods that require expertise to identify extremely small microsporidia spores (~ 2.0 µm). Our assays also improve upon existing molecular detection techniques for these microsporidia by incorporating an internal standard to control for false negative reactions.
Keywords: Encephalitozoon intestinalis; Enterocytozoon bieneusi; Immunofluorescence assay; Internal standard; PCR.
Conflict of interest statement
Compliance with ethical standardsOn behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures
References
-
- Alfa Cisse O, Ouattara A, Thellier M, Accoceberry I, Biligui S, Minta D, Doumbo O, Desportes-Livage I, Thera MA, Danis M, Datry A. Evaluation of an immunofluorescent-antibody test using monoclonal antibodies directed against Enterocytozoon bieneusi and Encephalitozoon intestinalis for diagnosis of intestinal microsporidiosis in Bamako (Mali) J Clin Microbiol. 2002;40:1715–1718. doi: 10.1128/JCM.40.5.1715-1718.2002. - DOI - PMC - PubMed
-
- Bergallo M, Costa C, Tarallo S, Daniele R, Merlino C, Segoloni GP, Negro Ponzi A, Cavallo R. Development of a quantitative-competitive PCR for quantification of human cytomegalovirus load and comparison with antigenaemia, viraemia and pp67 RNA detection by nucleic acid sequence-based amplification. Panminerva Med. 2006;48:119–127. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
