A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer
- PMID: 30956894
- PMCID: PMC6450396
- DOI: 10.1038/s41551-018-0254-6
A designer self-assembled supramolecule amplifies macrophage immune responses against aggressive cancer
Abstract
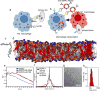
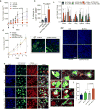
Effectively activating macrophages that can 'eat' cancer cells is challenging. In particular, cancer cells secrete macrophage colony stimulating factor (MCSF), which polarizes tumour-associated macrophages from an antitumour M1 phenotype to a pro-tumourigenic M2 phenotype. Also, cancer cells can express CD47, an 'eat me not' signal that ligates with the signal regulatory protein alpha (SIRPα) receptor on macrophages to prevent phagocytosis. Here, we show that a supramolecular assembly consisting of amphiphiles inhibiting the colony stimulating factor 1 receptor (CSF-1R) and displaying SIRPα-blocking antibodies with a drug-to-antibody ratio of 17,000 can disable both mechanisms. The supramolecule homes onto SIRPα on macrophages, blocking the CD47-SIRPα signalling axis while sustainedly inhibiting CSF-1R. The supramolecule enhances the M2-to-M1 repolarization within the tumour microenvironment, and significantly improves antitumour and antimetastatic efficacies in two aggressive animal models of melanoma and breast cancer, with respect to clinically available small-molecule and biologic inhibitors of CSF-1R signalling. Simultaneously blocking the CD47-SIRPα and MCSF-CSF-1R signalling axes may constitute a promising immunotherapy.
Conflict of interest statement
Competing interests: SS is a cofounder and holds equity in Akamara Therapeutics Inc. which is developing supramolecular therapeutics.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

