Unravelling hagfish slime
- PMID: 30958163
- PMCID: PMC6364663
- DOI: 10.1098/rsif.2018.0710
Unravelling hagfish slime
Abstract
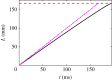
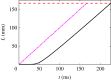
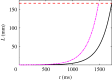
Hagfish slime is a unique predator defence material containing a network of long fibrous threads each ∼10 cm in length. Hagfish release the threads in a condensed coiled state known as skeins (∼100 µm), which must unravel within a fraction of a second to thwart a predator attack. Here we consider the hypothesis that viscous hydrodynamics can be responsible for this rapid unravelling, as opposed to chemical reaction kinetics alone. Our main conclusion is that, under reasonable physiological conditions, unravelling due to viscous drag can occur within a few hundred milliseconds, and is accelerated if the skein is pinned at a surface such as the mouth of a predator. We model a single skein unspooling as the fibre peels away due to viscous drag. We capture essential features by considering simplified cases of physiologically relevant flows and one-dimensional scenarios where the fibre is aligned with streamlines in either uniform or uniaxial extensional flow. The peeling resistance is modelled with a power-law dependence on peeling velocity. A dimensionless ratio of viscous drag to peeling resistance appears in the dynamical equations and determines the unraveling time scale. Our modelling approach is general and can be refined with future experimental measurements of peel strength for skein unravelling. It provides key insights into the unravelling process, offers potential answers to lingering questions about slime formation from threads and mucous vesicles, and will aid the growing interest in engineering similar bioinspired material systems.
Conflict of interest statement
We declare we have no competing interests.
Figures






References
-
- Waggett RJ, Buskey EJ. 2006. Calanoid copepod escape behavior in response to a visual predator. Mar. Biol. 150, 599–607. (10.1007/s00227-006-0384-3) - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

