Impact of Taurine on the proliferation and apoptosis of human cervical carcinoma cells and its mechanism
- PMID: 30958437
- PMCID: PMC6595772
- DOI: 10.1097/CM9.0000000000000162
Impact of Taurine on the proliferation and apoptosis of human cervical carcinoma cells and its mechanism
Abstract
Background: Cervical cancer has the fourth highest incidence and mortality rate of all cancers in women worldwide; it seriously harms their physical and mental health. The aim of this study was to observe the roles and preliminary mechanism of Taurine (Tau)-induced apoptosis in cervical cancer cells.
Methods: Cells from the human cervical cancer cell line SiHa were transfected with the recombinant plasmid pEGFP-N1-MST1 (mammalian sterile 20-like kinase 1); then, the cell proliferation activity was analyzed by the MTT assay, cell apoptosis by flow cytometry, and the related protein levels by Western blotting.
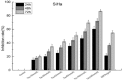
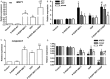
Results: Tau inhibited the proliferation of SiHa cells and induced apoptosis in these cells (the apoptotic rate was 21.95% in the Tau 160 mmol/L group and 30% in the Tau 320 mmol/L group), upregulated the expression of the MST1 (control, 0.53; Tau 40-320 mmol/L groups, 0.84-1.45) and Bax (control, 0.45; Tau 40-320 mmol/L groups, 0.64-1.51) proteins (P < 0.01), and downregulated the expression of Bcl-2 (control, 1.28, Tau 40-320 mmol/L groups, 0.93-0.47) (P < 0.01). The overexpression of MST1 promoted the apoptosis of SiHa cells, enhanced the apoptosis-inductive effects of Tau (P < 0.01), upregulated the expression of the proapoptotic proteins p73, p53, PUMA (p53 upregulated modulator of apoptosis), and caspase-3, and promoted the phosphorylation of YAP (Yes-associated protein).
Conclusions: Tau inhibited the proliferation and induced the apoptosis of cervical cancer SiHa cells. The MST1 protein plays an important role in the Tau-induced apoptosis of cervical cancer cells.
Figures




References
-
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. doi: 10.3322/caac.21262. - PubMed
-
- De Sanjosé S, Bruni L, Alemany L. HPV in genital cancers (at the exception of cervical cancer) and anal cancers. Presse Med 2014; 43:e423–e428. doi: 10.1016/j.lpm.2014.10.001. - PubMed
-
- Nelson EJ, Hughes J, Kulasingam SL. Spatial patterns of human papillomavirus-associated cancers within the state of Minnesota, 1998-2007. Spat Spatiotemporal Epidemiol 2014; 9:13–21. doi: 10.1016/j.sste.2014.02.003. - PubMed
-
- Munger K. Are selective estrogen receptor modulators (SERMs) a therapeutic option for HPV-associated cervical lesions and cancers? Am J Pathol 2014; 184:358–361. doi: 10.1016/j.ajpath.2013.11.005. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

