Cognitive-behavioral treatment with behavioral activation for smoking cessation: Randomized controlled trial
- PMID: 30958831
- PMCID: PMC6453447
- DOI: 10.1371/journal.pone.0214252
Cognitive-behavioral treatment with behavioral activation for smoking cessation: Randomized controlled trial
Abstract
Introduction: Behavioral Activation is a behavioral-based treatment that has been proposed as suitable for smoking cessation, as it simultaneously addresses reinforcement-related variables and also mood management. The aim of this study was to compare the effects of a cognitive-behavioral smoking cessation treatment with components of behavioral activation (SCBSCT-BA) with a standard cognitive-behavioral treatment (SCBSCT), and a wait-list control group (WL).
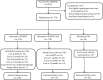
Method: The sample was comprised of 275 adults smokers (61.4% females, mean age = 45.36, SD = 10.96). After baseline assessment sessions, participants were randomized (ratio: 2.2.1.) to SCBSCT-BA, SCBSCT, or WL. Active groups received 8 weekly 1-hour face-to-face group sessions. Biochemically verified smoking abstinence and depressive symptoms were assessed at the end of treatment, and at 3-, 6-, and 12-month follow-ups.
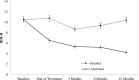
Results: Significant treatment effects in 7-dayspoint prevalence abstinence rates were found for both active groups at the end of treatment. Abstinence rates at 12-months follow-up were 30% for SCBSCT-BA, and 18% for SCBSCT. Using Multiple Imputation for missing data, regression analysis showed significantly greater ORs for the SCBSCT-BA condition (vs. SCBSCT) at the end of treatment and at 3-months follow-up. At 6-, and 12-months follow-ups, ORs for the SCBSCT-BA condition, although greater, did not reach statistical significance. Multilevel analysis showed that abstinence was related to reductions in depressive symptoms.
Conclusions: SCBSCT-BA obtained positive results at short and medium term. Participants who quit smoking experienced a significant reduction in depressive symptoms. Findings support the benefit of adding BA to a cognitive-behavioral smoking cessation treatment.
Trial registration: www.clinicaltrials.gov NCT02844595.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

