Optimizing live-animal bioluminescence imaging prediction of tumor burden in human prostate cancer xenograft models in SCID-NSG mice
- PMID: 30958914
- PMCID: PMC6668996
- DOI: 10.1002/pros.23802
Optimizing live-animal bioluminescence imaging prediction of tumor burden in human prostate cancer xenograft models in SCID-NSG mice
Abstract
Background: Noninvasive live-animal longitudinal monitoring of xenograft tumor growth and metastasis by bioluminescent imaging (BLI) has been widely reported in cancer biology and preclinical therapy literature, mainly in athymic nude mice. Our own experience at calibrating BLI readout with tumor weight/volume in human prostate cancer xenograft models in haired, SCID-NSG mice through intraprostatic (orthotopic) and subcutaneous (SC) inoculations revealed either nonexistent or poor correlation (coefficient of determination, R 2 = ~0.01-0.3). The present work examined several technical and biological factors to improve BLI utility.
Methods: After ruling out promoter-luciferase (luc) specificity and luc gene loss in the cell inoculum with LNCaP-AR-luc cells expressing an androgen receptor (AR) and tagged with AR-responsive probasin promoter-luc gene, we evaluated different routes of d-luciferin administration, imaging time during the day, charge-coupled device camera image acquisition settings, and hair removal methods to improve the imaging protocol. For most imaging sessions, BLI was carried out within the same day of tumor volume measurement. After necropsy, histological and immunohistochemical (IHC) analyses were performed on the tumors to evaluate necrosis and expression of luciferase and AR, respectively.
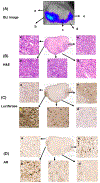
Results: Injection of d-luciferin by SC route, robust image-capture setting (30 000 counts and autoexposure), imaging in the morning and thorough hair removal resulted in a substantial improvement of R2 to ~0.6. Histological analyses confirmed the lack of BLI signal in necrotic tumor masses consistent with luciferase-mediated light emission only in oxygenated adenosine triphosphate-producing viable cells. IHC staining detected heterogeneous expression of luciferase tracking generally with AR expression in nonnecrotic tumor tissues.
Conclusions: Our body of work highlighted a framework to validate imaging protocols to ensure the acquisition of interpretable BLI data as an indicator of xenograft tumor burden. The vast tissue heterogeneity in prostate tumor xenografts and variable luciferase expression constrained this technology from achieving a high correlation.
Keywords: IVIS instrumental setting; bioluminescence; heterogeneous expression of luciferase; necrosis; prostate cancer.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Figures




References
-
- Valta M, Fagerlund K, Suominen M, Halleen J, Tuomela J. Importance of microenvironment in preclinical models of breast and prostate cancer. 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

