β-Lactamases and β-Lactamase Inhibitors in the 21st Century
- PMID: 30959050
- PMCID: PMC6723624
- DOI: 10.1016/j.jmb.2019.04.002
β-Lactamases and β-Lactamase Inhibitors in the 21st Century
Abstract
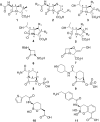
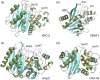
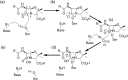
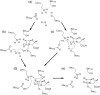
The β-lactams retain a central place in the antibacterial armamentarium. In Gram-negative bacteria, β-lactamase enzymes that hydrolyze the amide bond of the four-membered β-lactam ring are the primary resistance mechanism, with multiple enzymes disseminating on mobile genetic elements across opportunistic pathogens such as Enterobacteriaceae (e.g., Escherichia coli) and non-fermenting organisms (e.g., Pseudomonas aeruginosa). β-Lactamases divide into four classes; the active-site serine β-lactamases (classes A, C and D) and the zinc-dependent or metallo-β-lactamases (MBLs; class B). Here we review recent advances in mechanistic understanding of each class, focusing upon how growing numbers of crystal structures, in particular for β-lactam complexes, and methods such as neutron diffraction and molecular simulations, have improved understanding of the biochemistry of β-lactam breakdown. A second focus is β-lactamase interactions with carbapenems, as carbapenem-resistant bacteria are of grave clinical concern and carbapenem-hydrolyzing enzymes such as KPC (class A) NDM (class B) and OXA-48 (class D) are proliferating worldwide. An overview is provided of the changing landscape of β-lactamase inhibitors, exemplified by the introduction to the clinic of combinations of β-lactams with diazabicyclooctanone and cyclic boronate serine β-lactamase inhibitors, and of progress and strategies toward clinically useful MBL inhibitors. Despite the long history of β-lactamase research, we contend that issues including continuing unresolved questions around mechanism; opportunities afforded by new technologies such as serial femtosecond crystallography; the need for new inhibitors, particularly for MBLs; the likely impact of new β-lactam:inhibitor combinations and the continuing clinical importance of β-lactams mean that this remains a rewarding research area.
Keywords: antimicrobial resistance; carbapenemase; enzyme mechanism; metallo-β-lactamase; β-lactam.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Fleming A. On the antibacterial action of cultures of a penicillium with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929;10:226–236.
-
- Neu H.C. beta-Lactam antibiotics: structural relationships affecting in vitro activity and pharmacologic properties. Rev. Infect. Dis. 1986;8(Suppl. 3):S237–S259. - PubMed
-
- Brotzu G. 1948. Ricerche su di un nuovo antibiotico. Lavori dell'Istituto d'Igiene di Cagliari; pp. 4–18.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

