The emergent landscape of the mouse gut endoderm at single-cell resolution
- PMID: 30959515
- PMCID: PMC6724221
- DOI: 10.1038/s41586-019-1127-1
The emergent landscape of the mouse gut endoderm at single-cell resolution
Abstract
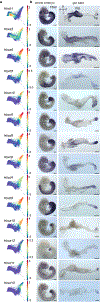
Here we delineate the ontogeny of the mammalian endoderm by generating 112,217 single-cell transcriptomes, which represent all endoderm populations within the mouse embryo until midgestation. We use graph-based approaches to model differentiating cells, which provides a spatio-temporal characterization of developmental trajectories and defines the transcriptional architecture that accompanies the emergence of the first (primitive or extra-embryonic) endodermal population and its sister pluripotent (embryonic) epiblast lineage. We uncover a relationship between descendants of these two lineages, in which epiblast cells differentiate into endoderm at two distinct time points-before and during gastrulation. Trajectories of endoderm cells were mapped as they acquired embryonic versus extra-embryonic fates and as they spatially converged within the nascent gut endoderm, which revealed these cells to be globally similar but retain aspects of their lineage history. We observed the regionalized identity of cells along the anterior-posterior axis of the emergent gut tube, which reflects their embryonic or extra-embryonic origin, and the coordinated patterning of these cells into organ-specific territories.
Conflict of interest statement
Competing interests
S.C.B and D.M.C. are employees and shareholders at 10x Genomics.
Figures










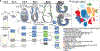
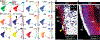



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

