Characteristics of ADHD Symptom Response/Remission in a Clinical Trial of Methylphenidate Extended Release
- PMID: 30959790
- PMCID: PMC6517933
- DOI: 10.3390/jcm8040461
Characteristics of ADHD Symptom Response/Remission in a Clinical Trial of Methylphenidate Extended Release
Abstract
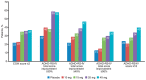
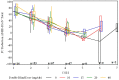
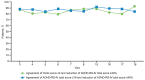
Clinical trials in attention-deficit/hyperactivity disorder (ADHD) have typically measured outcome using clinician ratings on the Attention-Deficit/Hyperactivity Disorder Rating Scale, Fourth Edition (ADHD-RS-IV) and the Clinical Global Impression-Improvement (CGI-I) scale. Remission has been defined as an endpoint score of less than or equal to 18 on the ADHD-RS-IV (or a mean score of 1). Responders have been defined as patients who achieve a CGI-I score of much or very much improved (1 or 2). There is a lack of agreement in the literature on what percent change in symptoms on the ADHD-RS-IV should be used to define improvement or remission. This study uses data from a clinical trial of a methylphenidate extended release (MPH-MLR; Aptensio XR®) phase III clinical trial to attempt to determine the percent change of symptoms that best corresponds with improvement and remission. Symptom remission at endpoint (ADHD-RS-IV total score ≤18) was most closely aligned with a ≥46% reduction in ADHD-RS-IV total score. Clinical improvement was most closely aligned with a ≥40% reduction in ADHD-RS-IV total score. The three different measures of outcome were strongly aligned during double blind and open label treatment, and were independent of subtype status. Our data suggest that at least 40% improvement in symptoms is needed to achieve a robust response at endpoint.
Keywords: attention deficit/hyperactivity disorder; central nervous system stimulants; methylphenidate; remission; response.
Conflict of interest statement
M. Weiss is a consultant and/or speaker bureau member for Eli Lilly, Janssen, Purdue, Rhodes Pharmaceuticals L.P., Shire, Akili, NLS Pharma, Cingulate Therapeutics. A. Childress receives research support from AEVI, Akili, Alcobra, Arbor, Forest Research Institute, Ironshore, Lilly USA, Lundbeck, Neos, Neurovance, Noven, Otsuka, Pearson, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, Supernus, and Tris; is a consultant and/or advisory board member for and received honoraria from AEVI, Akili, Arbor, Ironshore, Neos, Neurovance, NLS, Noven, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, Supernus, and Tris; has received payment for lectures from Arbor, Neos, Pfizer, Shire, and Tris; and has received writing assistance on projects from Arbor, Ironshore, Neos, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, and Tris. E. Nordbrock is a consultant for Rhodes Pharmaceuticals L.P. A.L. Adjei and R.J. Kupper are employees of Rhodes Pharmaceuticals L.P. G. Mattingly is a speaker for Alkermes, Allergan, Lundbeck, Merck, Neos, Otsuka, Shire, Sunovion, Takeda, and Vanda; is a consultant for Alkermes, Allergan, Forum, Lundbeck, Merck, Otsuka, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, Takeda, and Vanda; and has performed research for Akili, Alcobra, Alkermes, Allergan, Boehringer Ingelheim, Forum, Janssen, Medgenics, NLS, Reckitt Benckiser, Shire, Sunovion, Supernus, and Takeda.
Figures

References
-
- Swanson J.M., Kraemer H.C., Hinshaw S.P., Arnold L.E., Conners C.K., Abikoff H.B., Clevenger W., Davies M., Elliott G.R., Greenhill L.L., et al. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. - DOI - PubMed
-
- DuPaul G.J., Power T.J., Anastopoulos A.D., Reid R. ADHD Rating Scale–IV: Checklist, Norms, and Clinical Interpretation. Guilford Press; New York, NY, USA: 1998.
LinkOut - more resources
Full Text Sources

