Analysis of Association between Vitamin D Deficiency and Insulin Resistance
- PMID: 30959886
- PMCID: PMC6520736
- DOI: 10.3390/nu11040794
Analysis of Association between Vitamin D Deficiency and Insulin Resistance
Abstract
Recent evidence revealed extra skeleton activity of vitamin D, including prevention from cardiometabolic diseases and cancer development as well as anti-inflammatory properties. It is worth noting that vitamin D deficiency is very common and may be associated with the pathogenesis of insulin-resistance-related diseases, including obesity and diabetes. This review aims to provide molecular mechanisms showing how vitamin D deficiency may be involved in the insulin resistance formation. The PUBMED database and published reference lists were searched to find studies published between 1980 and 2019. It was identified that molecular action of vitamin D is involved in maintaining the normal resting levels of ROS and Ca2+, not only in pancreatic β-cells, but also in insulin responsive tissues. Both genomic and non-genomic action of vitamin D is directed towards insulin signaling. Thereby, vitamin D reduces the extent of pathologies associated with insulin resistance such as oxidative stress and inflammation. More recently, it was also shown that vitamin D prevents epigenetic alterations associated with insulin resistance and diabetes. In conclusion, vitamin D deficiency is one of the factors accelerating insulin resistance formation. The results of basic and clinical research support beneficial action of vitamin D in the reduction of insulin resistance and related pathologies.
Keywords: insulin resistance; insulin-responsive tissues; oxidative stress; pancreatic β-cells dysfunction; sub-inflammation; vitamin D.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
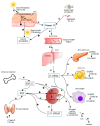
 —stimulation,
—stimulation,  —inhibition.
—inhibition.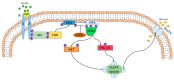

 —attenuation.
—attenuation.

 —activation.
—activation.
 —activation,
—activation,  —inhibition.
—inhibition.References
-
- Tabesh M., Azadbakht L., Faghihimani E., Tabesh M., Esmaillzadeh A. Effects of calcium-vitamin D co-supplementation on metabolic profiles in vitamin D insufficient people with type 2 diabetes: A randomised controlled clinical trial. Diabetologia. 2014;57:2038–2047. doi: 10.1007/s00125-014-3313-x. - DOI - PubMed
-
- Upreti V., Maitri V., Dhull P., Handa A., Prakash M.S., Behl A. Effect of oral vitamin D supplementation on glycemic control in patients with type 2 diabetes mellitus with coexisting hypovitaminosis D: A parellel group placebo controlled randomized controlled pilot study. Diabetes Metab. Syndr. 2018;12:509–512. doi: 10.1016/j.dsx.2018.03.008. - DOI - PubMed
-
- Barzegari M., Sarbakhsh P., Mobasseri M., Noshad H., Esfandiari A., Khodadadi B., Gargari B.P. The effects of vitamin D supplementation on lipid profiles and oxidative indices among diabetic nephropathy patients with marginal vitamin D status. Diabetes Metab. Syndr. 2019;13:542–547. doi: 10.1016/j.dsx.2018.11.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

