Morphological Transformation of Peptide Nanoassemblies through Conformational Transition of Core-forming Peptides
- PMID: 30960023
- PMCID: PMC6401806
- DOI: 10.3390/polym11010039
Morphological Transformation of Peptide Nanoassemblies through Conformational Transition of Core-forming Peptides
Abstract
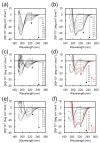
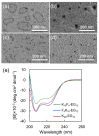
Morphological control of nanostructures that are composed of amphiphilic di- or tri-block molecules by external stimuli broadens their applications for molecular containers, nanoreactors, and controlled release materials. In this study, triblock amphiphiles comprising oligo(ethylene glycol), oligo(l-lysine), and tetra(l-phenylalanine) were prepared for the construction of nanostructures that can transform accompanying α-to-β transition of core-forming peptides. Circular dichroic (CD) measurements showed that the triblock amphiphiles adopted different secondary structures depending on the solvent environment: they adopt β-sheet structures in aqueous solution, while α-helix structures in 25% 2,2,2-trifluoroethanol (TFE) solution under basic pH conditions. Transmission electron microscopic (TEM) observation revealed that the triblock amphiphiles formed vesicle structures in 25% TFE aq. Solvent exchange from 25% TFE to water induced morphological transformation from vesicles to arc-shaped nanostructures accompanying α-β conformational transition. The transformable nanostructures may be useful as novel smart nanomaterials for molecular containers and micro reactors.
Keywords: aromatic peptides; morphological change; secondary structure; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Preparation and self-assembly behavior of β-sheet peptide-inserted amphiphilic block copolymer as a useful polymeric surfactant.Langmuir. 2013 Dec 17;29(50):15477-84. doi: 10.1021/la404328b. Epub 2013 Dec 6. Langmuir. 2013. PMID: 24289247
-
Conformational polymorphism of the amyloidogenic peptide homologous to residues 113-127 of the prion protein.Biophys J. 2003 Jul;85(1):473-83. doi: 10.1016/S0006-3495(03)74492-0. Biophys J. 2003. PMID: 12829502 Free PMC article.
-
1,1,1,3,3,3-Hexafluoro-2-propanol and 2,2,2-trifluoroethanol solvents induce self-assembly with different surface morphology in an aromatic dipeptide.Org Biomol Chem. 2014 Aug 28;12(32):6181-9. doi: 10.1039/c4ob00821a. Epub 2014 Jul 7. Org Biomol Chem. 2014. PMID: 24999600
-
Conformational studies of alanine-rich peptide using CD and FTIR spectroscopy.J Pept Sci. 2008 Mar;14(3):283-9. doi: 10.1002/psc.923. J Pept Sci. 2008. PMID: 17918765
-
pH-induced reversible conformational and morphological regulation of polyleucine grafted polyallylamine assembly in solution.Langmuir. 2005 Nov 22;21(24):11462-7. doi: 10.1021/la051594s. Langmuir. 2005. PMID: 16285826
Cited by
-
Investigation on the self-assembly of the NFL-TBS.40-63 peptide and its interaction with gold nanoparticles as a delivery agent for glioblastoma.Int J Pharm X. 2022 Sep 22;4:100128. doi: 10.1016/j.ijpx.2022.100128. eCollection 2022 Dec. Int J Pharm X. 2022. PMID: 36204592 Free PMC article.
References
-
- Li M.H., Keller P. Stimuli-responsive polymer vesicles. Soft Matter. 2009;5:927–937. doi: 10.1039/b815725a. - DOI
-
- Tian B., Tao X., Ren T., Weng Y., Lin X., Zhang Y., Tang X. Polypeptide-based vesicles: Formation, properties and application for drug delivery. J. Mater. Chem. 2012;22:17404–17414. doi: 10.1039/c2jm31806g. - DOI
LinkOut - more resources
Full Text Sources

