The Applications of Polymers in Solar Cells: A Review
- PMID: 30960127
- PMCID: PMC6401826
- DOI: 10.3390/polym11010143
The Applications of Polymers in Solar Cells: A Review
Abstract
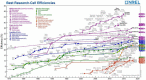
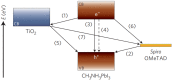
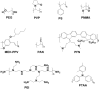
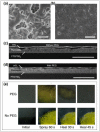
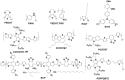
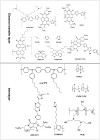
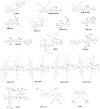
The emerging dye-sensitized solar cells, perovskite solar cells, and organic solar cells have been regarded as promising photovoltaic technologies. The device structures and components of these solar cells are imperative to the device's efficiency and stability. Polymers can be used to adjust the device components and structures of these solar cells purposefully, due to their diversified properties. In dye-sensitized solar cells, polymers can be used as flexible substrates, pore- and film-forming agents of photoanode films, platinum-free counter electrodes, and the frameworks of quasi-solid-state electrolytes. In perovskite solar cells, polymers can be used as the additives to adjust the nucleation and crystallization processes in perovskite films. The polymers can also be used as hole transfer materials, electron transfer materials, and interface layer to enhance the carrier separation efficiency and reduce the recombination. In organic solar cells, polymers are often used as donor layers, buffer layers, and other polymer-based micro/nanostructures in binary or ternary devices to influence device performances. The current achievements about the applications of polymers in solar cells are reviewed and analyzed. In addition, the benefits of polymers for solar cells, the challenges for practical application, and possible solutions are also assessed.
Keywords: applications; polymers; solar cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Sovacool B.K. National context derives concrens. Nat. Energy. 2018 doi: 10.1038/s41560-018-0246-5. - DOI
-
- Green M.A. The path to 25% silicon solar cell efficiency: History of silicon cell evolution. Prog. Photovolt Res. Appl. 2009;17:183–189. doi: 10.1002/pip.892. - DOI
-
- Razykov T.M., Ferekides C.S., Morel D., Stefanakos E., Ullal H.S., Upadhyaya H.M. Solar photovoltaic electricity: Current status and future prospects. Sol. Energy. 2011;85:1580–1608. doi: 10.1016/j.solener.2010.12.002. - DOI
-
- Hubbard S.M., Cress C.D., Bailey C.G., Raffaelle R.P., Bailey S.G., Wilt D.M. Effect of strain compensation on quantum dot enhanced GaAs solar cells. Appl. Phys. Lett. 2008;92:123512. doi: 10.1063/1.2903699. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

