Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, III: Extended Study on Effect of Catalyst Type on Molecular Weight Increase
- PMID: 30960422
- PMCID: PMC6473661
- DOI: 10.3390/polym11030438
Solid-State Polymerization of Poly(Ethylene Furanoate) Biobased Polyester, III: Extended Study on Effect of Catalyst Type on Molecular Weight Increase
Abstract
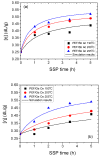
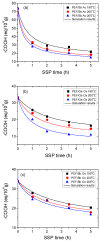
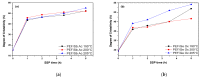
In this study, the synthesis of poly(ethylene furanoate) (PEF), catalyzed by five different catalysts-antimony acetate (III) (Sb Ac), zirconium (IV) isopropoxide isopropanal (Zr Is Ip), antimony (III) oxide (Sb Ox), zirconium (IV) 2,4-pentanedionate (Zr Pe) and germanium (IV) oxide (Ge Ox)-via an industrially common combination of melt polymerization and subsequent solid-state polymerization (SSP) is presented. In all reactions, proper amounts of 2,5-dimethylfuran-dicarboxylate (DMFD) and ethylene glycol (EG) in a molar ratio of DMFD/EG= 1/2 and 400 ppm of catalyst were used. Polyester samples were subjected to SSP procedure, under vacuum application, at different reaction times (1, 2, 3.5, and 5 h) and temperatures of 190, 200, and 205 °C. Carboxyl end-groups concentration (⁻COOH), intrinsic viscosity (IV), and thermal properties, via differential scanning calorimetry (DSC), were measured for all resultant polymers to study the effect of the used catalysts on the molecular weight increase of PEF during SSP process. As was expected, it was found that with increasing the SSP time and temperature, the intrinsic viscosity and the average molecular weight of PEF steadily increased. In contrast, the number of carboxyl end-groups content showed the opposite trend as intrinsic viscosity, that is, gradually decreasing during SSP time and temperature increase. It is worthy to note that thanks to the SSP process an obvious and continuous enhancement in the thermal properties of the prepared PEF samples was attained, in which their melting temperatures (Tm) and degree of crystallinity (Xc) increase progressively with increasing of reaction time and temperature. To predict the time evolution of polymers IV, as well as the hydroxyl and carboxyl content of PEF polyesters during the SSP, a simple kinetic model was developed. From both the theoretical simulation results and the experimental measurements, it was demonstrated that surely the Zr Is Ip catalyst shows the best catalytic characteristics compared to all other used catalysts herein, that is, leading in reducing-in a spectacular way-the activation energy of the involved both transesterification and esterification reactions during SSP.
Keywords: catalysts; poly(ethylene furanoate); polyester; solid-state polycondensation; thermal properties.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









References
-
- Gandini A. Polymers from Renewable Resources: A Challenge for the Future of Macromolecular Materials. Macromolecules. 2008;41:9491–9504. doi: 10.1021/ma801735u. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

