Hyaluronic Acid in the Third Millennium
- PMID: 30960626
- PMCID: PMC6403654
- DOI: 10.3390/polym10070701
Hyaluronic Acid in the Third Millennium
Abstract
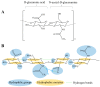
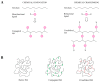
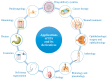
Since its first isolation in 1934, hyaluronic acid (HA) has been studied across a variety of research areas. This unbranched glycosaminoglycan consisting of repeating disaccharide units of N-acetyl-d-glucosamine and d-glucuronic acid is almost ubiquitous in humans and in other vertebrates. HA is involved in many key processes, including cell signaling, wound reparation, tissue regeneration, morphogenesis, matrix organization and pathobiology, and has unique physico-chemical properties, such as biocompatibility, biodegradability, mucoadhesivity, hygroscopicity and viscoelasticity. For these reasons, exogenous HA has been investigated as a drug delivery system and treatment in cancer, ophthalmology, arthrology, pneumology, rhinology, urology, aesthetic medicine and cosmetics. To improve and customize its properties and applications, HA can be subjected to chemical modifications: conjugation and crosslinking. The present review gives an overview regarding HA, describing its history, physico-chemical, structural and hydrodynamic properties and biology (occurrence, biosynthesis (by hyaluronan synthases), degradation (by hyaluronidases and oxidative stress), roles, mechanisms of action and receptors). Furthermore, both conventional and recently emerging methods developed for the industrial production of HA and its chemical derivatization are presented. Finally, the medical, pharmaceutical and cosmetic applications of HA and its derivatives are reviewed, reporting examples of HA-based products that currently are on the market or are undergoing further investigations.
Keywords: biological activity; cosmetic; crosslinking; drug delivery; food-supplement; functionalization; hyaluronan applications; hyaluronan derivatives; hyaluronan synthases; hyaluronic acid; hyaluronidases; physico-chemical properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Boeriu C.G., Springer J., Kooy F.K., van den Broek L.A.M., Eggink G. Production methods for hyaluronan. Int. J. Carbohydr. Chem. 2013;2013:14. doi: 10.1155/2013/624967. - DOI
-
- Meyer K., Palmer J.W. The polysaccharide of the vitrous humor. J. Biol. Chem. 1934;107:629–634.
-
- Kendall F.E., Heidelberger M., Dawson M.H. A serologically inactive polysaccharide elaborated by mucoid strains of group a hemolytic streptococcus. J. Biol. Chem. 1937;118:61–69.
-
- Boas N.F. Isolation of hyaluronic acid from the cock’s comb. J. Biol. Chem. 1949;181:573–575. - PubMed
-
- Kaye M.A., Stacey M. Observations on the chemistry of hyaluronic acid. Biochem. J. 1950;2:13. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

