Stimuli-Responsive Polypeptides for Biomedical Applications
- PMID: 30960755
- PMCID: PMC6404075
- DOI: 10.3390/polym10080830
Stimuli-Responsive Polypeptides for Biomedical Applications
Abstract
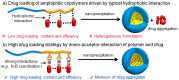
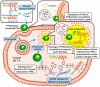
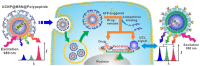
Stimuli-responsive polypeptides have gained attention because desirable bioactive properties can be easily imparted to them while keeping their biocompatibility and biodegradability intact. In this review, we summarize the most recent advances in various stimuli-responsive polypeptides (pH, reduction, oxidation, glucose, adenosine triphosphate (ATP), and enzyme) over the past five years. Various synthetic strategies exploited for advanced polypeptide-based materials are introduced, and their applicability in biomedical fields is discussed. The recent polypeptides imparted with new stimuli-responsiveness and their novel chemical and physical properties are explained in this review.
Keywords: drug and gene delivery systems; polypeptides-based materials; stimuli-responsiveness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

