How Green is Your Plasticizer?
- PMID: 30960759
- PMCID: PMC6403783
- DOI: 10.3390/polym10080834
How Green is Your Plasticizer?
Abstract
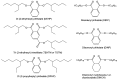
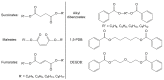
Plasticizers are additives that are used to impart flexibility to polymer blends and improve their processability. Plasticizers are typically not covalently bound to the polymers, allowing them to leach out over time, which results in human exposure and environmental contamination. Phthalates, in particular, have been the subject of increasing concern due to their established ubiquity in the environment and their suspected negative health effects, including endocrine disrupting and anti-androgenic effects. As there is mounting pressure to find safe replacement compounds, this review addresses the design and experimental elements that should be considered in order for a new or existing plasticizer to be considered green. Specifically, a multi-disciplinary and holistic approach should be taken which includes toxicity testing (both in vitro and in vivo), biodegradation testing (with attention to metabolites), as well as leaching studies. Special consideration should also be given to the design stages of producing a new molecule and the synthetic and scale-up processes should also be optimized. Only by taking a multi-faceted approach can a plasticizer be considered truly green.
Keywords: additive; biodegradation; leaching; metabolites; phthalate; plasticizer; toxicity.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- Braun D., Cherdron H., Ritter H. Polymer Synthesis: Theory and Practice: Fundamentals, Methods, Experiments. Springer; Heidelberg, Germany: 2001. pp. 105–110.
-
- Wilkes C.E., Summers J.W., Daniels C.A., Berard M.T. PVC Handbook. Hanser; Cincinnati, OH, USA: 2005.
-
- Sears J.K., Darby J.R. The Technology of Plasticizers. John Wiley; New York, NY, USA: 1982.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

