Synthesis of Poly(ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP
- PMID: 30960783
- PMCID: PMC6403792
- DOI: 10.3390/polym10080858
Synthesis of Poly(ε-caprolactone)-Based Miktoarm Star Copolymers through ROP, SA ATRC, and ATRP
Abstract
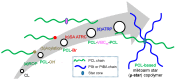
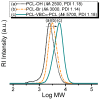
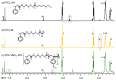
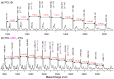
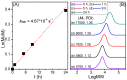
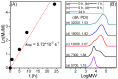
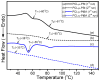
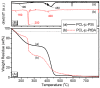
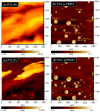
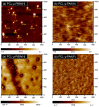
The synthesis of novel branched/star copolymers which possess unique physical properties is highly desirable. Herein, a novel strategy was demonstrated to synthesize poly(ε-caprolactone) (PCL) based miktoarm star (μ-star) copolymers by combining ring-opening polymerization (ROP), styrenics-assisted atom transfer radical coupling (SA ATRC), and atom transfer radical polymerization (ATRP). From the analyses of gel permeation chromatography (GPC), proton nuclear magnetic resonance (¹H NMR), and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS), well-defined PCL-μ-PSt (PSt: polystyrene), and PCL-μ-PtBA (PtBA: poly(tert-butyl acrylate) μ-star copolymers were successfully obtained. By using atomic force microscopy (AFM), interestingly, our preliminary examinations of the μ-star copolymers showed a spherical structure with diameters of ca. 250 and 45 nm, respectively. We successfully employed combinations of synthetic techniques including ROP, SA ATRC, and ATRP with high effectiveness to synthesize PCL-based μ-star copolymers.
Keywords: atom transfer radical polymerization; miktoarm star copolymers; ring-opening polymerization; styrenics-assisted atom transfer radical coupling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bielawski C.W., Grubbs R.H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 2007;32:1–29. doi: 10.1016/j.progpolymsci.2006.08.006. - DOI
-
- Mecerreyes D., Jerome R., Dubois P. Novel macromolecular architectures based on aliphatic polyesters: Relevance of the coordination-insertion ring-opening polymerization. Adv. Polym. Sci. 1999;147:1–59.
-
- Braunecker W.A., Matyjaszewski K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007;32:93–146. doi: 10.1016/j.progpolymsci.2006.11.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

