Effects of Achyranthes bidentata Polysaccharides on Intestinal Morphology, Immune Response, and Gut Microbiome in Yellow Broiler Chickens Challenged with Escherichia coli K88
- PMID: 30961158
- PMCID: PMC6401798
- DOI: 10.3390/polym10111233
Effects of Achyranthes bidentata Polysaccharides on Intestinal Morphology, Immune Response, and Gut Microbiome in Yellow Broiler Chickens Challenged with Escherichia coli K88
Abstract
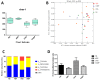
The present study was conducted to investigate the effects of dietary Achyranthes bidentata polysaccharide (ABPS) supplementation on performance, immune response, intestinal mucosal morphology, and gut microbiome in yellow-feathered broilers challenged with Escherichia coli K88. A 2 × 2 factorial design was used for the trial. Two hundred and forty one-day-old female broilers were randomly assigned to four treatments: (1) negative-control broilers were fed by a basal diet and saline (NG); (2) positive-control broilers were fed by a basal diet and orally challenged with 10⁸ CFU E. coli K88 (CNG); (3) ABP group broilers were fed by a basal diet containing ABPS (500 mg/kg of feed) and saline; (4) CABP group broilers were fed by a basal diet containing ABPS (500 mg/kg of feed) and orally challenged with 10⁸ CFU E. coli K88. Growth performance, serum biochemical indexes, immune responses, intestinal mucosal morphology, and cecal microbial community structure were evaluated. The ABP group had greatest body weight (BW), average daily body weight gain (ADG), and intestinal villus height compared to other treatments (p < 0.05). The CABP group had a higher villus height/crypt depth ratio (V/C) compared with other treatments (p < 0.05). The expression levels of NF-κB were lower in the ABP group. The CNG group had higher Escherichia coli and Enterococcus contents in cecal samples compared to other treatments (p < 0.05). Serum glucose, uric acid, TNF-α, and Secretory Immunoglobulin A (S-IgA) levels were higher in broilers challenged with E. coli (p < 0.001) than that with saline. Broilers challenged with E. coli had reduced taxa richness in the cecal samples. Sequencing of 16S rRNA genes in cecal samples revealed that a lower proportion of Firmicutes and a higher proportion of Proteobacteria were detected in the broilers challenged with E. coli. Compared with the controls, dietary ABPS supplementation increased serum total protein, albumin, and S-IgA levels, but decreased serum glucose, uric acid, and TNF-α levels in broilers (p < 0.05). Diet supplemented with ABPS increased the Firmicutes/Bacteroidetes ratio and the abundance of Ruminococcaceae and Lachnospiraceae, and altered cecal microbiota community structure. These results suggest that ABPS can promote growth performance and improve intestinal morphology and microbiota community structure in broilers challenged with E. coli K88.
Keywords: Achyranthes bidentata polysaccharides; Escherichia coli K88; cecal microbiota; immune response; intestinal mucosal morphology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- He C.L., Fu B.D., Shen H.Q., Jiang X.L., Zhang C.S., Wu S.C., Zhu W., Wei X.B. Xiang-Qi-Tang Increases Avian Pathogenic Escherichia coli-Induced Survival Rate and Regulates Serum Levels of Tumor Necrosis Factor Alpha, Interleukin-1 and Soluble Endothelial Protein C Receptor in Chicken. Biol. Pharm. Bull. 2011;34:379–382. doi: 10.1248/bpb.34.379. - DOI - PubMed
-
- Baurhoo B., Letellier A., Zhao X., Ruiz-Feria C.A. Cecal Populations of Lactobacilli and Bifidobacteria and Escherichia coli Populations after In Vivo Escherichia coli Challenge in Birds Fed Diets with Purified Lignin or Mannanoligosaccharides. Poult. Sci. 2007;86:2509–2516. doi: 10.3382/ps.2007-00136. - DOI - PubMed
-
- Zhang L., Cao G.T., Zeng X.F., Zhou L., Ferket P.R., Xiao Y.P., Chen A.G., Yang C.M. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013;93:46–53. doi: 10.3382/ps.2013-03412. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

