Multiscale Modeling of Structure, Transport and Reactivity in Alkaline Fuel Cell Membranes: Combined Coarse-Grained, Atomistic and Reactive Molecular Dynamics Simulations
- PMID: 30961214
- PMCID: PMC6401961
- DOI: 10.3390/polym10111289
Multiscale Modeling of Structure, Transport and Reactivity in Alkaline Fuel Cell Membranes: Combined Coarse-Grained, Atomistic and Reactive Molecular Dynamics Simulations
Abstract
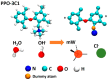
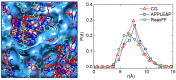
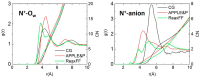
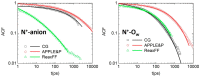
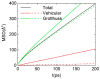
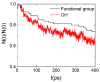
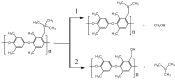
In this study, molecular dynamics (MD) simulations of hydrated anion-exchange membranes (AEMs), comprised of poly(p-phenylene oxide) (PPO) polymers functionalized with quaternary ammonium cationic groups, were conducted using multiscale coupling between three different models: a high-resolution coarse-grained (CG) model; Atomistic Polarizable Potential for Liquids, Electrolytes and Polymers (APPLE&P); and ReaxFF. The advantages and disadvantages of each model are summarized and compared. The proposed multiscale coupling utilizes the strength of each model and allows sampling of a broad spectrum of properties, which is not possible to sample using any of the single modeling techniques. Within the proposed combined approach, the equilibrium morphology of hydrated AEM was prepared using the CG model. Then, the morphology was mapped to the APPLE&P model from equilibrated CG configuration of the AEM. Simulations using atomistic non-reactive force field allowed sampling of local hydration structure of ionic groups, vehicular transport mechanism of anion and water, and structure equilibration of water channels in the membrane. Subsequently, atomistic AEM configuration was mapped to ReaxFF reactive model to investigate the Grotthuss mechanism in the hydroxide transport, as well as the AEM chemical stability and degradation mechanisms. The proposed multiscale and multiphysics modeling approach provides valuable input for the materials-by-design of novel polymeric structures for AEMs.
Keywords: alkaline fuel cells; atomistic and coarse-grained models; multiscale molecular simulations; polymer membranes; reactive molecular simulations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Molecular Modeling in Anion Exchange Membrane Research: A Brief Review of Recent Applications.Molecules. 2022 Jun 2;27(11):3574. doi: 10.3390/molecules27113574. Molecules. 2022. PMID: 35684512 Free PMC article. Review.
-
High-Resolution Coarse-Grained Model of Hydrated Anion-Exchange Membranes that Accounts for Hydrophobic and Ionic Interactions through Short-Ranged Potentials.J Chem Theory Comput. 2017 Jan 10;13(1):245-264. doi: 10.1021/acs.jctc.6b00874. Epub 2016 Dec 7. J Chem Theory Comput. 2017. PMID: 28068769
-
Grotthuss versus Vehicular Transport of Hydroxide in Anion-Exchange Membranes: Insight from Combined Reactive and Nonreactive Molecular Simulations.J Phys Chem Lett. 2018 Feb 15;9(4):825-829. doi: 10.1021/acs.jpclett.8b00004. Epub 2018 Feb 5. J Phys Chem Lett. 2018. PMID: 29390610
-
Role of cationic groups on structural and dynamical correlations in hydrated quaternary ammonium-functionalized poly(p-phenylene oxide)-based anion exchange membranes.Phys Chem Chem Phys. 2018 Jul 25;20(29):19350-19362. doi: 10.1039/c8cp02211a. Phys Chem Chem Phys. 2018. PMID: 29993087
-
Computational Approaches to Alkaline Anion-Exchange Membranes for Fuel Cell Applications.Membranes (Basel). 2022 Oct 27;12(11):1051. doi: 10.3390/membranes12111051. Membranes (Basel). 2022. PMID: 36363606 Free PMC article. Review.
Cited by
-
Hydroxide Diffusion in Functionalized Cylindrical Nanopores as Idealized Models of Anion Exchange Membrane Environments: An Ab Initio Molecular Dynamics Study.J Phys Chem C Nanomater Interfaces. 2023 Feb 2;127(6):2792-2804. doi: 10.1021/acs.jpcc.2c05747. eCollection 2023 Feb 16. J Phys Chem C Nanomater Interfaces. 2023. PMID: 36968146 Free PMC article.
-
Molecular Modeling in Anion Exchange Membrane Research: A Brief Review of Recent Applications.Molecules. 2022 Jun 2;27(11):3574. doi: 10.3390/molecules27113574. Molecules. 2022. PMID: 35684512 Free PMC article. Review.
-
Perspective: Morphology and ion transport in ion-containing polymers from multiscale modeling and simulations.Front Chem. 2022 Aug 19;10:981508. doi: 10.3389/fchem.2022.981508. eCollection 2022. Front Chem. 2022. PMID: 36059884 Free PMC article. Review.
-
Molecular Modeling Investigations of Sorption and Diffusion of Small Molecules in Glassy Polymers.Membranes (Basel). 2019 Aug 8;9(8):98. doi: 10.3390/membranes9080098. Membranes (Basel). 2019. PMID: 31398889 Free PMC article. Review.
References
-
- Yanagi H., Fukuta K. Anion Exchange Membrane and Ionomer for Alkaline Membrane Fuel Cells (AMFCs) ECS Trans. 2008;16:257–262. doi: 10.1149/1.2981860. - DOI
-
- Merle G., Wessling M., Nijmeijer K. Anion Exchange Membranes for Alkaline Fuel Cells: A Review. J. Membr. Sci. 2011;377:1–35. doi: 10.1016/j.memsci.2011.04.043. - DOI
-
- Hagesteijn K.F., Jiang S., Ladewig B.P. A Review of The Synthesis and Characterization of Anion Exchange Membranes. J. Mater. Sci. 2018;53:11131–11150. doi: 10.1007/s10853-018-2409-y. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

