Plasticizers Derived from Biomass Resources: A Short Review
- PMID: 30961228
- PMCID: PMC6401779
- DOI: 10.3390/polym10121303
Plasticizers Derived from Biomass Resources: A Short Review
Abstract
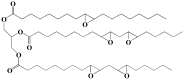
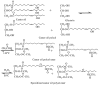
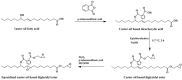
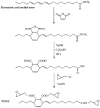
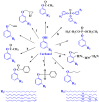
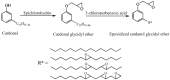
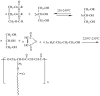
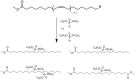
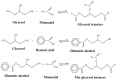
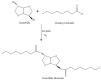
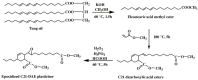
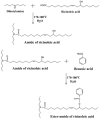
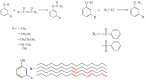
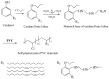
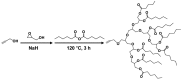
With rising environmental concerns and depletion of petrochemical resources, biomass-based chemicals have been paid more attention. Polyvinyl chloride (PVC) plasticizers derived from biomass resources (vegetable oil, cardanol, vegetable fatty acid, glycerol and citric acid) have been widely studied to replace petroleum-based o-phthalate plasticizers. These bio-based plasticizers mainly include epoxidized plasticizer, polyester plasticizer, macromolecular plasticizer, flame retardant plasticizer, citric acid ester plasticizer, glyceryl ester plasticizer and internal plasticizer. Bio-based plasticizers with the advantages of renewability, degradability, hypotoxicity, excellent solvent resistant extraction and plasticizing performances make them potential to replace o-phthalate plasticizers partially or totally. In this review, we classify different types of bio-based plasticizers according to their chemical structure and function, and highlight recent advances in multifunctional applications of bio-based plasticizers in PVC products. This study will increase the interest of researchers in bio-based plasticizers and the development of new ideas in this field.
Keywords: biomass resources; plasticizer; polyvinyl chloride; review.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
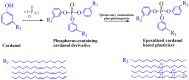


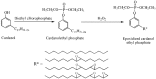
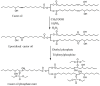



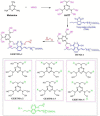


References
-
- Rahman M., Brazel C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004;29:1223–1248. doi: 10.1016/j.progpolymsci.2004.10.001. - DOI
-
- Choi J.S., Park W.H. Effect of biodegradable plasticizers on thermal and mechanical properties of poly(3-hydroxybutyrate) Polym. Test. 2004;23:455–460. doi: 10.1016/j.polymertesting.2003.09.005. - DOI
-
- Wingborg N., Eldsäter C. 2,2-Dinitro-1,3-Bis-Nitrooxy-Propane (NPN): A New Energetic Plasticizer. Propellants Explos. Pyrotech. 2002;27:314–319. doi: 10.1002/prep.200290000. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

