Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis
- PMID: 30961539
- PMCID: PMC6454693
- DOI: 10.1186/s12876-019-0961-9
Diagnostic accuracy of controlled attenuation parameter (CAP) as a non-invasive test for steatosis in suspected non-alcoholic fatty liver disease: a systematic review and meta-analysis
Abstract
Background: Controlled attenuation parameter (CAP) is a non-invasive method for diagnosing hepatic steatosis. Despite good diagnostic performance, clinical application of CAP is limited due to the influences of covariates. Here, a systematic review on the performance of CAP in the diagnosis and staging of hepatic steatosis in NAFLD patients was performed.
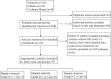
Methods: The sensitivity, specificity, diagnostic odds ratio (DOR) and area under receiver operating characteristics (AUROC) curves of the pooled data for CAP in diagnosing and staging the mild (Stage 1), moderate (Stage 2) and severe (Stage 3) steatosis in NAFLD patients were assessed. The clinical utility of CAP was evaluated by Fagan plot. Heterogeneity was explored using subgroup analysis.
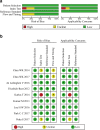
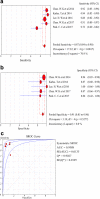
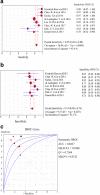
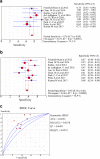
Results: Nine studies involving 1297 patients with liver biopsy-proven NAFLD were analyzed. The pooled sensitivity of CAP in detecting mild hepatic steatosis was 87% with a specificity of 91% and a DOR of 84.35. The pooled sensitivity of CAP in detecting moderate hepatic steatosis was 85% with a specificity of 74% and a DOR of 21.28. For severe steatosis, the pooled sensitivity was 76% with a specificity of 58% and a DOR of 4.70. The mean AUROC value for CAP in the diagnosis of mild, moderate, and severe steatosis was 0.96, 0.82 and 0.70, respectively. A subgroup analysis indicated that variation in the geographic regions, cutoffs, age and body mass index (BMI) could be the potential sources of heterogeneity in the diagnosis of moderate to severe steatosis.
Conclusions: CAP should be cautiously considered as a non-invasive substitute for liver biopsy in clinical practice.
Keywords: Controlled attenuation parameter (CAP); Hepatic steatosis, diagnostic accuracy; Non-alcoholic fatty liver disease (NAFLD); Transient elastography.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis.EClinicalMedicine. 2022 Jul 10;51:101547. doi: 10.1016/j.eclinm.2022.101547. eCollection 2022 Sep. EClinicalMedicine. 2022. PMID: 35844772 Free PMC article.
-
Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease.J Gastroenterol Hepatol. 2014;29(7):1470-6. doi: 10.1111/jgh.12557. J Gastroenterol Hepatol. 2014. PMID: 24548002
-
Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease.J Gastroenterol Hepatol. 2016 Apr;31(4):848-55. doi: 10.1111/jgh.13219. J Gastroenterol Hepatol. 2016. PMID: 26514665
-
A meta-analysis on the diagnostic performance of magnetic resonance imaging and transient elastography in nonalcoholic fatty liver disease.Eur J Clin Invest. 2021 Feb;51(2):e13446. doi: 10.1111/eci.13446. Epub 2020 Dec 9. Eur J Clin Invest. 2021. PMID: 33128454
-
Diagnostic accuracy of ultrasound-guided attenuation parameter as a noninvasive test for steatosis in non-alcoholic fatty liver disease.J Med Ultrason (2001). 2021 Oct;48(4):471-480. doi: 10.1007/s10396-021-01123-0. Epub 2021 Aug 20. J Med Ultrason (2001). 2021. PMID: 34415481 Review.
Cited by
-
First-in-human diagnostic study of hepatic steatosis with computed ultrasound tomography in echo mode.Commun Med (Lond). 2023 Dec 9;3(1):176. doi: 10.1038/s43856-023-00409-3. Commun Med (Lond). 2023. PMID: 38071269 Free PMC article.
-
Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis.EClinicalMedicine. 2022 Jul 10;51:101547. doi: 10.1016/j.eclinm.2022.101547. eCollection 2022 Sep. EClinicalMedicine. 2022. PMID: 35844772 Free PMC article.
-
Associations between Phase Angle Values Obtained by Bioelectrical Impedance Analysis and Nonalcoholic Fatty Liver Disease in an Overweight Population.Can J Gastroenterol Hepatol. 2020 Aug 4;2020:8888405. doi: 10.1155/2020/8888405. eCollection 2020. Can J Gastroenterol Hepatol. 2020. PMID: 32832491 Free PMC article.
-
Accuracy of controlled attenuation parameter compared with ultrasound for detecting hepatic steatosis in children with severe obesity.Eur Radiol. 2021 Mar;31(3):1588-1596. doi: 10.1007/s00330-020-07245-2. Epub 2020 Sep 10. Eur Radiol. 2021. PMID: 32910234 Free PMC article.
-
Compensated Advanced Chronic Liver Disease and Steatosis in Patients with Type 2 Diabetes as Assessed through Shear Wave Measurements and Attenuation Measurements.Biomedicines. 2024 Jan 30;12(2):323. doi: 10.3390/biomedicines12020323. Biomedicines. 2024. PMID: 38397925 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

