A Novel Endogenous Damage Signal, CSF-2, Activates Multiple Beneficial Functions of Adipose Tissue-Derived Mesenchymal Stem Cells
- PMID: 30962162
- PMCID: PMC6554530
- DOI: 10.1016/j.ymthe.2019.03.010
A Novel Endogenous Damage Signal, CSF-2, Activates Multiple Beneficial Functions of Adipose Tissue-Derived Mesenchymal Stem Cells
Abstract
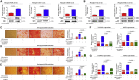
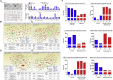
The major challenges of current mesenchymal stem cell (MSC)-based therapeutics are their low differentiation potential into specialized cell types and their homing ability to sites of injury. Therefore, many researchers have directed their efforts toward finding a novel stimulatory factor that can significantly enhance the therapeutic effects of MSCs. Colony-stimulating factor 2 (CSF-2) is previously known as a hematopoietic growth factor involved in the differentiation of various myeloid cells from hematopoietic progenitor cells. In addition to this canonical hematopoietic function, we identified for the first time that CSF-2 is actively secreted by stem cells, in response to various types of injuries, as an endogenous damage signal that promotes the therapeutic effects of MSCs by enhancing their multi-lineage differentiation and migratory capacities, possibly through its receptor CD116. Our results also revealed that CSF-2 exerts its stimulatory effects on MSCs via PI3K/Akt- and/or FAK/ERK1/2-signaling pathways. More importantly, we also found that MSCs stimulated with CSF-2 show markedly enhanced differentiation and migratory capacities and subsequent in vivo therapeutic effects in an endometrial ablation animal model. Collectively, our findings provide compelling evidence for a novel non-hematopoietic function of CSF-2 in promoting multiple beneficial functions of MSCs via a non-canonical mechanism as an endogenous damage signal.
Keywords: Akt; CSF-2; ERK1/2; MSCs; differentiation; growth; migration; stemness.
Copyright © 2019 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures






References
-
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. - PubMed
-
- Ha Y., Kim Y.S., Cho J.M., Yoon S.H., Park S.R., Yoon D.H., Kim E.Y., Park H.C. Role of granulocyte-macrophage colony-stimulating factor in preventing apoptosis and improving functional outcome in experimental spinal cord contusion injury. J. Neurosurg. Spine. 2005;2:55–61. - PubMed
-
- Pastore S., Fanales-Belasio E., Albanesi C., Chinni L.M., Giannetti A., Girolomoni G. Granulocyte macrophage colony-stimulating factor is overproduced by keratinocytes in atopic dermatitis. Implications for sustained dendritic cell activation in the skin. J. Clin. Invest. 1997;99:3009–3017. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

