Nur77 Links Chronic Antigen Stimulation to B Cell Tolerance by Restricting the Survival of Self-Reactive B Cells in the Periphery
- PMID: 30962292
- PMCID: PMC6504591
- DOI: 10.4049/jimmunol.1801565
Nur77 Links Chronic Antigen Stimulation to B Cell Tolerance by Restricting the Survival of Self-Reactive B Cells in the Periphery
Abstract
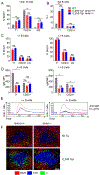
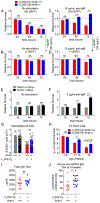
Nur77 (Nr4a1) belongs to a small family of orphan nuclear receptors that are rapidly induced by BCR stimulation, yet little is known about its function in B cells. We have previously characterized a reporter of Nr4a1 transcription, Nur77-eGFP, in which GFP expression faithfully detects Ag encounter by B cells in vitro and in vivo. In this study, we report that Nur77 expression correlates with the degree of self-reactivity, counterselection, and anergy among individual B cell clones from two distinct BCR transgenic mouse models but is dispensable for all of these tolerance mechanisms. However, we identify a role for Nur77 in restraining survival of self-reactive B cells in the periphery under conditions of competition for a limited supply of the survival factor BAFF. We find that Nur77 deficiency results in the progressive accumulation of self-reactive B cells in the mature repertoire with age and is sufficient to break B cell tolerance in VH3H9 H chain transgenic mice. We thus propose that Nur77 is upregulated in self-reactive B cells in response to chronic Ag stimulation and selectively restricts the survival of these cells, gradually pruning self-reactivity from the mature repertoire to impose a novel layer of peripheral B cell tolerance.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures






References
-
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, and Nussenzweig MC. 2003. Predominant autoantibody production by early human B cell precursors. Science 301: 1374–1377. - PubMed
-
- Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu H-B, and Cyster JG. 2004. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20: 441–453. - PubMed
-
- Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, and Brink R. 2004. Excess BAFF Rescues Self-Reactive B Cells from Peripheral Deletion and Allows Them to Enter Forbidden Follicular and Marginal Zone Niches. Immunity 20: 785–798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

