Pulmonary Pharmacokinetics of Oseltamivir Carboxylate in Rats after Nebulization or Intravenous Administration of Its Prodrug, Oseltamivir Phosphate
- PMID: 30962337
- PMCID: PMC6535510
- DOI: 10.1128/AAC.00074-19
Pulmonary Pharmacokinetics of Oseltamivir Carboxylate in Rats after Nebulization or Intravenous Administration of Its Prodrug, Oseltamivir Phosphate
Abstract
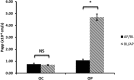
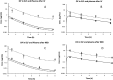
The aim of this study was to investigate the pharmacokinetics of oseltamivir phosphate, a prodrug, and its active moiety in plasma and lung after its nebulization and intravenous administration in rats. Only 2% of prodrug was converted into active moiety presystematically, attesting to a low advantage of oseltamivir phosphate nebulization, suggesting that oseltamivir phosphate nebulization is not a good option to obtain a high exposure of the active moiety at the infection site within lung.
Keywords: lung; nebulization; oseltamivir; pharmacokinetics; prodrug.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Biopharmaceutical Characterization of Nebulized Antimicrobial Agents in Rats: 5. Oseltamivir Carboxylate.Antimicrob Agents Chemother. 2016 Jul 22;60(8):5085-7. doi: 10.1128/AAC.00909-16. Print 2016 Aug. Antimicrob Agents Chemother. 2016. PMID: 27297482 Free PMC article.
-
Clinical pharmacokinetics of oseltamivir and its active metabolite oseltamivir carboxylate after oral administration in horses.J Vet Med Sci. 2007 Mar;69(3):293-6. doi: 10.1292/jvms.69.293. J Vet Med Sci. 2007. PMID: 17409647
-
Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations.J Antimicrob Chemother. 2010 Apr;65 Suppl 2(Suppl 2):ii5-ii10. doi: 10.1093/jac/dkq015. J Antimicrob Chemother. 2010. PMID: 20215135 Free PMC article. Review.
-
Simulation of the Pharmacokinetics of Oseltamivir and Its Active Metabolite in Normal Populations and Patients with Hepatic Cirrhosis Using Physiologically Based Pharmacokinetic Modeling.AAPS PharmSciTech. 2020 Mar 3;21(3):98. doi: 10.1208/s12249-020-1638-y. AAPS PharmSciTech. 2020. PMID: 32128656
-
Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802.Clin Pharmacokinet. 1999 Dec;37(6):471-84. doi: 10.2165/00003088-199937060-00003. Clin Pharmacokinet. 1999. PMID: 10628898 Review.
Cited by
-
Treatment of respiratory viral infections through inhalation therapeutics: Challenges and opportunities.Pulm Pharmacol Ther. 2022 Dec;77:102170. doi: 10.1016/j.pupt.2022.102170. Epub 2022 Oct 12. Pulm Pharmacol Ther. 2022. PMID: 36240985 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

