Lysocins: Bioengineered Antimicrobials That Deliver Lysins across the Outer Membrane of Gram-Negative Bacteria
- PMID: 30962344
- PMCID: PMC6535517
- DOI: 10.1128/AAC.00342-19
Lysocins: Bioengineered Antimicrobials That Deliver Lysins across the Outer Membrane of Gram-Negative Bacteria
Abstract
The prevalence of multidrug-resistant Pseudomonas aeruginosa has stimulated development of alternative therapeutics. Bacteriophage peptidoglycan hydrolases, termed lysins, represent an emerging antimicrobial option for targeting Gram-positive bacteria. However, lysins against Gram-negatives are generally deterred by the outer membrane and their inability to work in serum. One solution involves exploiting evolved delivery systems used by colicin-like bacteriocins (e.g., S-type pyocins of P. aeruginosa) to translocate through the outer membrane. Following surface receptor binding, colicin-like bacteriocins form Tol- or TonB-dependent translocons to actively import bactericidal domains through outer membrane protein channels. With this understanding, we developed lysocins, which are bioengineered
Keywords: ESKAPE; Pseudomonas aeruginosa; antibiotic resistance; antimicrobial; endolysin; lysin; lysocin; peptidoglycan hydrolase; protein delivery.
Copyright © 2019 American Society for Microbiology.
Figures

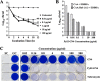


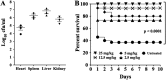
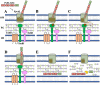
Similar articles
-
Impact of FiuA Outer Membrane Receptor Polymorphism on the Resistance of Pseudomonas aeruginosa toward Peptidoglycan Lipid II-Targeting PaeM Pyocins.J Bacteriol. 2019 Jun 10;201(13):e00164-19. doi: 10.1128/JB.00164-19. Print 2019 Jul 1. J Bacteriol. 2019. PMID: 30988031 Free PMC article.
-
PlyKp104, a Novel Phage Lysin for the Treatment of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Other Gram-Negative ESKAPE Pathogens.Antimicrob Agents Chemother. 2023 May 17;67(5):e0151922. doi: 10.1128/aac.01519-22. Epub 2023 Apr 26. Antimicrob Agents Chemother. 2023. PMID: 37098944 Free PMC article.
-
A Colicin M-Type Bacteriocin from Pseudomonas aeruginosa Targeting the HxuC Heme Receptor Requires a Novel Immunity Partner.Appl Environ Microbiol. 2018 Aug 31;84(18):e00716-18. doi: 10.1128/AEM.00716-18. Print 2018 Sep 15. Appl Environ Microbiol. 2018. PMID: 29980560 Free PMC article.
-
Colicin import into E. coli cells: a model system for insights into the import mechanisms of bacteriocins.Biochim Biophys Acta. 2014 Aug;1843(8):1717-31. doi: 10.1016/j.bbamcr.2014.04.010. Epub 2014 Apr 16. Biochim Biophys Acta. 2014. PMID: 24746518 Review.
-
Turning Over a New Leaf: Bacteriocins Going Green.Trends Microbiol. 2018 Jan;26(1):1-2. doi: 10.1016/j.tim.2017.11.001. Epub 2017 Nov 14. Trends Microbiol. 2018. PMID: 29150081 Review.
Cited by
-
Development of sensitizer peptide-fused endolysin Lys1S-L9P acting against multidrug-resistant gram-negative bacteria.Front Microbiol. 2023 Nov 23;14:1296796. doi: 10.3389/fmicb.2023.1296796. eCollection 2023. Front Microbiol. 2023. PMID: 38075915 Free PMC article.
-
PaP1, a Broad-Spectrum Lysin-Derived Cationic Peptide to Treat Polymicrobial Skin Infections.Front Microbiol. 2022 Mar 10;13:817228. doi: 10.3389/fmicb.2022.817228. eCollection 2022. Front Microbiol. 2022. PMID: 35369520 Free PMC article.
-
Nanobody-Targeted Conditional Antimicrobial Therapeutics.ACS Nano. 2025 Mar 18;19(10):9958-9970. doi: 10.1021/acsnano.4c16007. Epub 2025 Mar 5. ACS Nano. 2025. PMID: 40044143 Free PMC article.
-
Treating Bacterial Infections with Bacteriophage-Based Enzybiotics: In Vitro, In Vivo and Clinical Application.Antibiotics (Basel). 2021 Dec 6;10(12):1497. doi: 10.3390/antibiotics10121497. Antibiotics (Basel). 2021. PMID: 34943709 Free PMC article. Review.
-
Mining of Gram-Negative Surface-Active Enzybiotic Candidates by Sequence-Based Calculation of Physicochemical Properties.Front Microbiol. 2021 May 25;12:660403. doi: 10.3389/fmicb.2021.660403. eCollection 2021. Front Microbiol. 2021. PMID: 34113327 Free PMC article.
References
-
- McCaughey LC, Josts I, Grinter R, White P, Byron O, Tucker NP, Matthews JM, Kleanthous C, Whitchurch CB, Walker D. 2016. Discovery, characterization and in vivo activity of pyocin SD2, a protein antibiotic from Pseudomonas aeruginosa. Biochem J 473:2345–2358. doi:10.1042/BCJ20160470. - DOI - PMC - PubMed
-
- Hattemer A, Hauser A, Diaz M, Scheetz M, Shah N, Allen JP, Porhomayon J, El-Solh AA. 2013. Bacterial and clinical characteristics of health care- and community-acquired bloodstream infections due to Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:3969–3975. doi:10.1128/AAC.02467-12. - DOI - PMC - PubMed
-
- WHO. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva, Switzerland: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

