Widespread soil bacterium that oxidizes atmospheric methane
- PMID: 30962365
- PMCID: PMC6486757
- DOI: 10.1073/pnas.1817812116
Widespread soil bacterium that oxidizes atmospheric methane
Erratum in
-
Correction for Tveit et al., Widespread soil bacterium that oxidizes atmospheric methane.Proc Natl Acad Sci U S A. 2025 Jan 21;122(3):e2426002122. doi: 10.1073/pnas.2426002122. Epub 2025 Jan 9. Proc Natl Acad Sci U S A. 2025. PMID: 39786943 Free PMC article. No abstract available.
Abstract
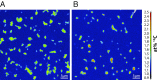
The global atmospheric level of methane (CH4), the second most important greenhouse gas, is currently increasing by ∼10 million tons per year. Microbial oxidation in unsaturated soils is the only known biological process that removes CH4 from the atmosphere, but so far, bacteria that can grow on atmospheric CH4 have eluded all cultivation efforts. In this study, we have isolated a pure culture of a bacterium, strain MG08 that grows on air at atmospheric concentrations of CH4 [1.86 parts per million volume (p.p.m.v.)]. This organism, named Methylocapsa gorgona, is globally distributed in soils and closely related to uncultured members of the upland soil cluster α. CH4 oxidation experiments and 13C-single cell isotope analyses demonstrated that it oxidizes atmospheric CH4 aerobically and assimilates carbon from both CH4 and CO2 Its estimated specific affinity for CH4 (a0s) is the highest for any cultivated methanotroph. However, growth on ambient air was also confirmed for Methylocapsa acidiphila and Methylocapsa aurea, close relatives with a lower specific affinity for CH4, suggesting that the ability to utilize atmospheric CH4 for growth is more widespread than previously believed. The closed genome of M. gorgona MG08 encodes a single particulate methane monooxygenase, the serine cycle for assimilation of carbon from CH4 and CO2, and CO2 fixation via the recently postulated reductive glycine pathway. It also fixes dinitrogen and expresses the genes for a high-affinity hydrogenase and carbon monoxide dehydrogenase, suggesting that atmospheric CH4 oxidizers harvest additional energy from oxidation of the atmospheric trace gases carbon monoxide (0.2 p.p.m.v.) and hydrogen (0.5 p.p.m.v.).
Keywords: USC alpha; filter cultivation; isolate; methane; trace gases.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- IPCC . Climate Change 2013–The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the IPCC. Cambridge Univ Press; Cambridge, UK: 2013.
-
- Dlugokencky EJ, Nisbet EG, Fisher R, Lowry D. Global atmospheric methane: Budget, changes and dangers. Philos Trans A Math Phys Eng Sci. 2011;369:2058–2072. - PubMed
-
- Kirschke S, et al. Three decades of global methane sources and sinks. Nat Geosci. 2013;6:813–823.
-
- Conrad R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ Microbiol Rep. 2009;1:285–292. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

