Cytochrome b 5 Is an Obligate Electron Shuttle Protein for Syringyl Lignin Biosynthesis in Arabidopsis
- PMID: 30962392
- PMCID: PMC6588299
- DOI: 10.1105/tpc.18.00778
Cytochrome b 5 Is an Obligate Electron Shuttle Protein for Syringyl Lignin Biosynthesis in Arabidopsis
Abstract
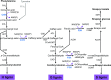
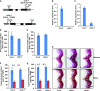
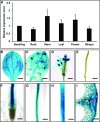
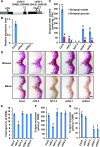
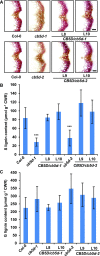
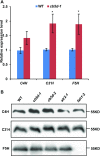
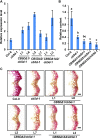
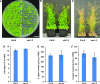
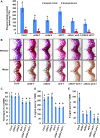
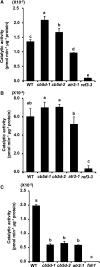
Angiosperms have evolved the metabolic capacity to synthesize p-hydroxyphenyl, guaiacyl (G), and syringyl (S) lignin subunits in their cell walls to better adapt to the harsh terrestrial environment. The structural characteristics of lignin subunits are essentially determined by three cytochrome P450-catalzyed reactions. NADPH-dependent cytochrome P450 oxidoreductase (CPR) is commonly regarded as the electron carrier for P450-catalyzed reactions during monolignol biosynthesis. Here, we show that cytochrome b 5 isoform D (CB5D) is an indispensable electron shuttle protein specific for S-lignin biosynthesis. Arabidopsis (Arabidopsis thaliana) CB5D localizes to the endoplasmic reticulum membrane and physically associates with monolignol P450 enzymes. Disrupting CB5D in Arabidopsis resulted in a >60% reduction in S-lignin subunit levels but no impairment in G-lignin formation compared with the wild type, which sharply contrasts with the impaired G- and S-lignin synthesis observed after disrupting ATR2, encoding Arabidopsis CPR. The defective S-lignin synthesis in cb5d mutants was rescued by the expression of the gene encoding CB5D but not with mutant CB5D devoid of its electron shuttle properties. Disrupting ATR2 suppressed the catalytic activity of both cinnamic acid 4-hydroxylase and ferulate 5-hydroxylase (F5H), but eliminating CB5D specifically depleted the latter's activity. Therefore, CB5D functions as an obligate electron shuttle intermediate that specifically augments F5H-catalyzed reactions, thereby controlling S-lignin biosynthesis.
© 2019 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Backes W.L., Kelley R.W. (2003). Organization of multiple cytochrome P450s with NADPH-cytochrome P450 reductase in membranes. Pharmacol. Ther. 98: 221–233. - PubMed
-
- Boerjan W., Ralph J., Baucher M. (2003). Lignin biosynthesis. Annu. Rev. Plant Biol. 54: 519–546. - PubMed
-
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. - PubMed
-
- Cowley A.B., Altuve A., Kuchment O., Terzyan S., Zhang X., Rivera M., Benson D.R. (2002). Toward engineering the stability and hemin-binding properties of microsomal cytochromes b5 into rat outer mitochondrial membrane cytochrome b5: Examining the influence of residues 25 and 71. Biochemistry 41: 11566–11581. - PubMed
-
- Dailey H.A., Strittmatter P. (1979). Modification and identification of cytochrome b5 carboxyl groups involved in protein-protein interaction with cytochrome b5 reductase. J. Biol. Chem. 254: 5388–5396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

