On resin click-chemistry-mediated synthesis of novel enkephalin analogues with potent anti-nociceptive activity
- PMID: 30962495
- PMCID: PMC6453917
- DOI: 10.1038/s41598-019-42289-5
On resin click-chemistry-mediated synthesis of novel enkephalin analogues with potent anti-nociceptive activity
Abstract
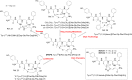
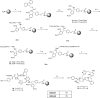
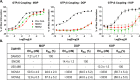
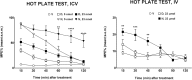
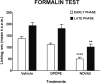
Here, we report the chemical synthesis of two DPDPE analogues 7a (NOVA1) and 7b (NOVA2). This entailed the solid-phase synthesis of two enkephalin precursor chains followed by a CuI-catalyzed azide-alkyne cycloaddition, with the aim of improving in vivo analgesic efficacy versus DPDPE. NOVA2 showed good affinity and selectivity for the μ-opioid receptor (KI of 59.2 nM, EC50 of 12.9 nM, EMax of 87.3%), and long lasting anti-nociceptive effects in mice when compared to DPDPE.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Assessment of stereoselectivity of trimethylphenylalanine analogues of delta-opioid [D-Pen(2),D-Pen(5)]-enkephalin.J Neurochem. 2000 Jul;75(1):424-35. doi: 10.1046/j.1471-4159.2000.0750424.x. J Neurochem. 2000. PMID: 10854288
-
[L-Ala3]DPDPE: a new enkephalin analog with a unique opioid receptor activity profile. Further evidence of delta-opioid receptor multiplicity.J Med Chem. 1994 May 27;37(11):1572-7. doi: 10.1021/jm00037a007. J Med Chem. 1994. PMID: 8201592
-
The metabolic evidence of synergistic interaction between DAMGO and DPDPE on undifferentiated SH-SY5Y cells.Neuroreport. 2001 Mar 26;12(4):845-9. doi: 10.1097/00001756-200103260-00044. Neuroreport. 2001. PMID: 11277594
-
Opioid peptide receptor studies. 9. Identification of a novel non-mu- non-delta-like opioid peptide binding site in rat brain.Peptides. 1998;19(6):1079-90. doi: 10.1016/s0196-9781(98)00046-1. Peptides. 1998. PMID: 9700759
-
CuAAC: An Efficient Click Chemistry Reaction on Solid Phase.ACS Comb Sci. 2016 Jan 11;18(1):1-14. doi: 10.1021/acscombsci.5b00087. Epub 2015 Dec 21. ACS Comb Sci. 2016. PMID: 26652044 Review.
Cited by
-
LC-MS Based Analysis and Biological Properties of Pseudocedrela kotschyi (Schweinf.) Harms Extracts: A Valuable Source of Antioxidant, Antifungal, and Antibacterial Compounds.Antioxidants (Basel). 2021 Oct 2;10(10):1570. doi: 10.3390/antiox10101570. Antioxidants (Basel). 2021. PMID: 34679706 Free PMC article.
-
Discovery of Orexant and Anorexant Agents with Indazole Scaffold Endowed with Peripheral Antiedema Activity.Biomolecules. 2019 Sep 16;9(9):492. doi: 10.3390/biom9090492. Biomolecules. 2019. PMID: 31527522 Free PMC article.
-
Bone-Targeted Extracellular Vesicles from Mesenchymal Stem Cells for Osteoporosis Therapy.Int J Nanomedicine. 2020 Oct 15;15:7967-7977. doi: 10.2147/IJN.S263756. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33116512 Free PMC article.
-
Phenolic Profile, Toxicity, Enzyme Inhibition, In Silico Studies, and Antioxidant Properties of Cakile maritima Scop. (Brassicaceae) from Southern Portugal.Plants (Basel). 2020 Jan 22;9(2):142. doi: 10.3390/plants9020142. Plants (Basel). 2020. PMID: 31979182 Free PMC article.
-
A Survey of Molecular Imaging of Opioid Receptors.Molecules. 2019 Nov 19;24(22):4190. doi: 10.3390/molecules24224190. Molecules. 2019. PMID: 31752279 Free PMC article. Review.
References
-
- Weber SJ, et al. Assessment of an in vitro blood-brain barrier model using several [Met5]enkephalin opioid analogs. J. Pharmacol. Exp. Ther. 1993;266:1649–1655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

