Distinct cortical-amygdala projections drive reward value encoding and retrieval
- PMID: 30962632
- PMCID: PMC6486448
- DOI: 10.1038/s41593-019-0374-7
Distinct cortical-amygdala projections drive reward value encoding and retrieval
Abstract
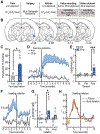
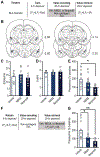
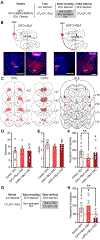
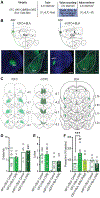
The value of an anticipated rewarding event is a crucial component of the decision to engage in its pursuit. But little is known of the networks responsible for encoding and retrieving this value. By using biosensors and pharmacological manipulations, we found that basolateral amygdala (BLA) glutamatergic activity tracks and mediates encoding and retrieval of the state-dependent incentive value of a palatable food reward. Projection-specific, bidirectional chemogenetic and optogenetic manipulations revealed that the orbitofrontal cortex (OFC) supports the BLA in these processes. Critically, the function of ventrolateral and medial OFC→BLA projections is doubly dissociable. Whereas lateral OFC→BLA projections are necessary and sufficient for encoding of the positive value of a reward, medial OFC→BLA projections are necessary and sufficient for retrieving this value from memory. These data reveal a new circuit for adaptive reward valuation and pursuit and provide insight into the dysfunction in these processes that characterizes myriad psychiatric diseases.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no biomedical financial interests or potential conflicts of interest.
Figures
Comment in
-
Anatomical specialties for value information.Nat Neurosci. 2019 May;22(5):685-686. doi: 10.1038/s41593-019-0387-2. Nat Neurosci. 2019. PMID: 30962631 No abstract available.
References
-
- Dickinson A & Balleine BW Motivational control over goal-directed action. Animal Learning and Behavior 22, 1–18 (1994).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

