A randomized controlled trial of povidone-iodine/dexamethasone ophthalmic suspension for acute viral conjunctivitis
- PMID: 30962674
- PMCID: PMC6433103
- DOI: 10.2147/OPTH.S191275
A randomized controlled trial of povidone-iodine/dexamethasone ophthalmic suspension for acute viral conjunctivitis
Abstract
Purpose: To evaluate the clinical safety and efficacy of povidone-iodine (PVP-I) 0.6%/dexamethasone (DEX) 0.1% ophthalmic suspension vs vehicle in patients with clinically suspected acute viral conjunctivitis.
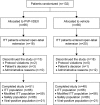
Patients and methods: This was a randomized, double-masked, parallel-group, vehicle-controlled study. Adults with a clinical diagnosis of suspected acute viral conjunctivitis were randomized 1:1 to PVP-I/DEX ophthalmic suspension or vehicle bilaterally four times daily for 5 days (Days 1-5). Evaluation was on Days 1, 3 (+1-day window), and 6 (+1). Patients with signs of acute viral conjunctivitis at the Day 6 visit received open-label PVP-I/DEX for five additional days and were evaluated on Day 11-14. The primary efficacy endpoint was clinical resolution of acute viral conjunctivitis in the study eye at the Day 6 visit.
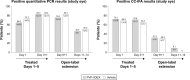
Results: Overall, 132 patients were randomized and received treatment (PVP-I/DEX, n=66; vehicle, n=66); 38 patients continued into the open-label portion of the study. Not enough patients with confirmed adenoviral conjunctivitis (n=32/132) were enrolled to assess the primary endpoint, although there were some efficacy trends in the PVP-I/DEX group for global clinical score (sum of watery conjunctival discharge and bulbar conjunctival redness). There were no serious treatment-emergent adverse events (TEAEs) and no patients discontinued due to a TEAE. In the masked phase, 56.1% of patients receiving PVP-I/DEX experienced at least one TEAE vs 43.9% in the vehicle group; 78.9% of patients in the open-label phase experienced at least one TEAE. Most TEAEs were mild in severity.
Conclusion: PVP-I/DEX ophthalmic suspension administered for ≤14 days had a favorable safety profile and was generally well tolerated.
Keywords: adenoviral conjunctivitis; dexamethasone; povidone-iodine; randomized controlled trial.
Conflict of interest statement
Disclosure Jay S Pepose: consultant/advisor for AcuFocus, Inc., Bausch & Lomb, BRIM Biotech, Johnson & Johnson, Noveome, Shire, Sun Pharma, and TearLab; and has equity interest in AcuFocus, Inc., Mimetogen, Ocunexis, Okogen, and Stuart Pharmaceuticals. Wenlei Liu is an employee of Shire, a Takeda company, and owns stock/stock options in Takeda. Abhijit Narvekar and Reza Haque were employees of Shire, a Takeda company, and owned stock/stock options in Shire at the time of this work.
Figures

References
-
- Sambursky RP, Fram N, Cohen EJ. The prevalence of adenoviral conjunctivitis at the Wills Eye Hospital emergency room. Optometry. 2007;78(5):236–239. - PubMed
-
- Woodland RM, Darougar S, Thaker U, et al. Causes of conjunctivitis and keratoconjunctivitis in Karachi, Pakistan. Trans R Soc Trop Med Hyg. 1992;86(3):317–320. - PubMed
-
- O’Brien TP, Jeng BH, McDonald M, Raizman MB. Acute conjunctivitis: truth and misconceptions. Curr Med Res Opin. 2009;25(8):1953–1961. - PubMed
LinkOut - more resources
Full Text Sources

