Single- and multiple-dose tolerability, safety, pharmacokinetics, and pharmacodynamics of the dual endothelin receptor antagonist aprocitentan in healthy adult and elderly subjects
- PMID: 30962677
- PMCID: PMC6435120
- DOI: 10.2147/DDDT.S199051
Single- and multiple-dose tolerability, safety, pharmacokinetics, and pharmacodynamics of the dual endothelin receptor antagonist aprocitentan in healthy adult and elderly subjects
Abstract
Background: Aprocitentan is an orally active, dual endothelin (ET) receptor antagonist developed for the treatment of hypertension in which, despite available treatments, a medical need exists for drugs with a new mechanism of action.
Subjects and methods: In this study, the single- and multiple-dose tolerability, safety, pharmacokinetics (PK), and pharmacodynamics of up to 600 mg (single doses) and 100 mg once a day (qd; multiple doses) of aprocitentan were investigated in healthy male and female subjects. The effect of age on the tolerability and PK parameters was investigated at a dose of 100 mg qd.
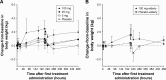
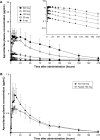
Results: Aprocitentan was well tolerated across all doses. No serious adverse events (AEs) occurred. The most frequently reported AE was headache. Small increases in body weight were recorded in subjects receiving 100 mg qd. Plasma concentration-time profiles of aprocitentan were similar after single- and multiple-dose administration, and support a qd dosing regimen based on a half-life of 44 hours. After multiple doses, PK was dose proportional. Accumulation at steady state, reached by Day 8, was 3-fold. Only minor differences in exposure between healthy females and males, healthy elderly and adult subjects, and fed and fasted conditions were observed. Plasma ET-1 concentrations, reflecting ETB receptor antagonism, significantly increased with doses ≥25 mg. Time-matched analysis of electrocardiogram (ECG) parameters did not suggest drug-induced ECG effects. Exposure-response analysis indicated no QTc prolongations at plasma levels up to 10 µg/mL.
Conclusion: Aprocitentan was well tolerated in healthy subjects with a PK profile favorable for qd dosing.
Keywords: aprocitentan; endothelin; endothelin receptor antagonist; first-in-human study; pharmacodynamics; pharmacokinetics.
Conflict of interest statement
Disclosure JD is a fellow of the American College of Clinical Pharmacology. PNS and JD are current employees of Idorsia Pharmaceuticals Ltd and former employees of Actelion Pharmaceuticals Ltd. MM is a current employee of Idorsia Pharmaceuticals Ltd. MKK was the principal investigator of the study that was sponsored by Actelion Pharmaceuticals Ltd. The authors report no other conflicts of interest in this work.
Figures



Similar articles
-
Multiple-Dose Pharmacokinetics, Safety, and Tolerability of Aprocitentan, a Dual Endothelin Receptor Antagonist, in Healthy Japanese and Caucasian Subjects.Clin Pharmacol Drug Dev. 2021 Jul;10(7):718-725. doi: 10.1002/cpdd.881. Epub 2020 Oct 15. Clin Pharmacol Drug Dev. 2021. PMID: 33063477 Clinical Trial.
-
Effects of Multiple-Dose Administration of Aprocitentan on the Pharmacokinetics of Rosuvastatin.Clin Pharmacol Drug Dev. 2020 Nov;9(8):995-1002. doi: 10.1002/cpdd.815. Epub 2020 Jun 27. Clin Pharmacol Drug Dev. 2020. PMID: 32592633 Clinical Trial.
-
Single-Dose Pharmacokinetics and Tolerability of Aprocitentan, a Dual Endothelin Receptor Antagonist, in Subjects with Severe Renal Function Impairment.Clin Drug Investig. 2019 Nov;39(11):1117-1123. doi: 10.1007/s40261-019-00837-x. Clin Drug Investig. 2019. PMID: 31435905 Clinical Trial.
-
Aprocitentan, A Dual Endothelin Receptor Antagonist Under Development for the Treatment of Resistant Hypertension.Cardiol Ther. 2021 Dec;10(2):397-406. doi: 10.1007/s40119-021-00233-7. Epub 2021 Jul 12. Cardiol Ther. 2021. PMID: 34251649 Free PMC article. Review.
-
Pressing Update: Aprocitentan for the Treatment of Hypertension.Ann Pharmacother. 2025 Apr;59(4):364-370. doi: 10.1177/10600280241273218. Epub 2024 Sep 4. Ann Pharmacother. 2025. PMID: 39229973 Review.
Cited by
-
Single-dose pharmacokinetics, safety, and tolerability of the dual endothelin receptor antagonist aprocitentan in subjects with moderate hepatic impairment.Sci Rep. 2022 Nov 9;12(1):19067. doi: 10.1038/s41598-022-22470-z. Sci Rep. 2022. PMID: 36352054 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Aprocitentan in the Treatment of Hypertension: A Meta-Analysis of Evidence from Randomized Controlled Trials.Rev Cardiovasc Med. 2025 Jan 20;26(1):25909. doi: 10.31083/RCM25909. eCollection 2025 Jan. Rev Cardiovasc Med. 2025. PMID: 39867183 Free PMC article.
-
Current and future strategies for targeting the endothelin pathway in cardiovascular disease.Nat Cardiovasc Res. 2023 Nov;2(11):972-990. doi: 10.1038/s44161-023-00347-2. Epub 2023 Nov 2. Nat Cardiovasc Res. 2023. PMID: 39196099 Review.
-
Effect of Multiple-Dose Aprocitentan Administration on the Pharmacokinetics of Midazolam in Healthy Male Subjects.Eur J Drug Metab Pharmacokinet. 2020 Apr;45(2):227-234. doi: 10.1007/s13318-019-00590-8. Eur J Drug Metab Pharmacokinet. 2020. PMID: 31773427 Clinical Trial.
-
Chinese Guidelines for the Prevention and Treatment of Hypertension (2024 revision).J Geriatr Cardiol. 2025 Jan 28;22(1):1-149. doi: 10.26599/1671-5411.2025.01.008. J Geriatr Cardiol. 2025. PMID: 40151633 Free PMC article. No abstract available.
References
-
- Moser M, Setaro JF. Clinical practice. Resistant or difficult-to-control hypertension. N Engl J Med. 2006;355(4):385–392. - PubMed
-
- Mancia G, Fagard R, Narkiewicz K, et al. Task Force Members 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31(7):1281–1357. - PubMed
-
- Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

