Osteochondral repair using scaffolds with gradient pore sizes constructed with silk fibroin, chitosan, and nano-hydroxyapatite
- PMID: 30962685
- PMCID: PMC6435123
- DOI: 10.2147/IJN.S191627
Osteochondral repair using scaffolds with gradient pore sizes constructed with silk fibroin, chitosan, and nano-hydroxyapatite
Abstract
Background: One of the main problems associated with the development of osteochondral reparative materials is that the accurate imitation of the structure of the natural osteochondral tissue and fabrication of a suitable scaffold material for osteochondral repair are difficult. The long-term outcomes of single- or bilayered scaffolds are often unsatisfactory because of the absence of a progressive osteochondral structure. Therefore, only scaffolds with gradient pore sizes are suitable for osteochondral repair to achieve better proliferation and differentiation of the stem cells into osteochondral tissues to complete the repair of defects.
Methods: A silk fibroin (SF) solution, chitosan (CS) solution, and nano-hydroxyapatite (nHA) suspension were mixed at the same weight fraction to obtain osteochondral scaffolds with gradient pore diameters by centrifugation, freeze-drying, and chemical cross-linking.
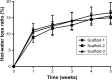
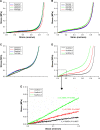
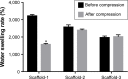
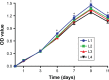
Results: The scaffolds prepared in this study are confirmed to have a progressive structure starting from the cartilage layer to bone layer, similar to that of the normal osteochondral tissues. The prepared scaffolds are cylindrical in shape and have high internal porosity. The structure consists of regular and highly interconnected pores with a progressively increasing pore distribution as well as a progressively changing pore diameter. The scaffold strongly absorbs water, and has a suitable degradation rate, sufficient space for cell growth and proliferation, and good resistance to compression. Thus, the scaffold can provide sufficient nutrients and space for cell growth, proliferation, and migration. Further, bone marrow mesenchymal stem cells seeded onto the scaffold closely attach to the scaffold and stably grow and proliferate, indicating that the scaffold has good biocompatibility with no cytotoxicity.
Conclusion: In brief, the physical properties and biocompatibility of our scaffolds fully comply with the requirements of scaffold materials required for osteochondral tissue engineering, and they are expected to become a new type of scaffolds with gradient pore sizes for osteochondral repair.
Keywords: bioscaffolds; bone marrow mesenchymal stem cells; osteochondral defect; tissue engineering.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures



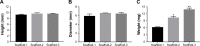
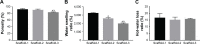

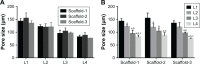


Similar articles
-
Silk fibroin/collagen and silk fibroin/chitosan blended three-dimensional scaffolds for tissue engineering.Eur J Orthop Surg Traumatol. 2015 Feb;25(2):243-9. doi: 10.1007/s00590-014-1515-z. Epub 2014 Aug 14. Eur J Orthop Surg Traumatol. 2015. PMID: 25118870
-
Preparation of SF-gel-CS-Hap bionic biphasic porous scaffolds and evaluation of physical, mechanical and biological properties.J Biomater Appl. 2025 Jul;40(1):61-81. doi: 10.1177/08853282251329591. Epub 2025 Mar 24. J Biomater Appl. 2025. PMID: 40123528
-
[Preparation and in vitro evaluation of tissue engineered osteochondral integration of multi-layered scaffold].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Apr 15;32(4):434-440. doi: 10.7507/1002-1892.201712038. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29806301 Free PMC article. Chinese.
-
Osteochondral Tissue Engineering: Scaffold Materials, Fabrication Techniques and Applications.Biotechnol J. 2025 Jan;20(1):e202400699. doi: 10.1002/biot.202400699. Biotechnol J. 2025. PMID: 39865414 Review.
-
Gradient scaffolds for osteochondral tissue engineering and regeneration.J Mater Chem B. 2020 Sep 23;8(36):8149-8170. doi: 10.1039/d0tb00688b. J Mater Chem B. 2020. PMID: 32776030 Review.
Cited by
-
Use of nanoparticles in skeletal tissue regeneration and engineering.Histol Histopathol. 2020 Apr;35(4):331-350. doi: 10.14670/HH-18-184. Epub 2019 Nov 13. Histol Histopathol. 2020. PMID: 31721139 Review.
-
Chitosan/Silk Fibroin Materials for Biomedical Applications-A Review.Polymers (Basel). 2022 Mar 26;14(7):1343. doi: 10.3390/polym14071343. Polymers (Basel). 2022. PMID: 35406217 Free PMC article. Review.
-
Recombinant Human Bone Morphogenic Protein-2 Immobilized Fabrication of Magnesium Functionalized Injectable Hydrogels for Controlled-Delivery and Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells in Femoral Head Necrosis Repair.Front Cell Dev Biol. 2021 Nov 25;9:723789. doi: 10.3389/fcell.2021.723789. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34900987 Free PMC article.
-
Production of chitosan scaffolds by lyophilization or electrospinning: which is better for peripheral nerve regeneration?Neural Regen Res. 2021 Jun;16(6):1093-1098. doi: 10.4103/1673-5374.300463. Neural Regen Res. 2021. PMID: 33269755 Free PMC article.
-
A Biomimetic Biphasic Scaffold Consisting of Decellularized Cartilage and Decalcified Bone Matrixes for Osteochondral Defect Repair.Front Cell Dev Biol. 2021 Feb 19;9:639006. doi: 10.3389/fcell.2021.639006. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33681223 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

