Predictors of achieving remission in schizophrenia patients treated with paliperidone palmitate 3-month formulation
- PMID: 30962688
- PMCID: PMC6435125
- DOI: 10.2147/NDT.S194264
Predictors of achieving remission in schizophrenia patients treated with paliperidone palmitate 3-month formulation
Abstract
Purpose: Long-acting injectable (LAI) antipsychotic paliperidone palmitate 3-month formulation (PP3M) is indicated in the United States for the treatment of schizophrenia only after adequate treatment with paliperidone palmitate 1-month formulation (PP1M) for ≥4 months. This analysis aimed to identify patient and disease characteristics during PP1M treatment associated with greater likelihood of achieving remission after transition to PP3M.
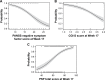
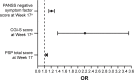
Methods: A post hoc analysis of a randomized, Phase III, double-blind, noninferiority trial of PP3M vs PP1M (ClinicalTrials.gov identifier: NCT01515423) was conducted in adult patients with schizophrenia. Patients achieving clinical stability after 17 weeks of open-label PP1M were randomized to 48 weeks of double-blind treatment with PP3M or PP1M. The primary objective of this exploratory post hoc analysis was to identify demographic and/or clinical variables associated with persistent remission after treatment with PP3M. Multiple logistic regression analysis identified the following significant predictors of remission: Positive and Negative Syndrome Scale (PANSS) Marder negative symptom factor score, Clinical Global Impression-Severity (CGI-S) total score, and Personal and Social Performance (PSP) total score.
Results: At double-blind baseline, a 1-point reduction in Marder negative symptom factor score was associated with a 20% increase in the odds of achieving remission after PP3M treatment; 1-point reduction in CGI-S was associated with a doubling in remission odds; and 7- and 10-point improvements in PSP scores, respectively, were associated with 42% and 65% increases in remission odds.
Conclusion: Patients with early clinically meaningful improvements in disease symptoms and severity while establishing stable PP1M dosage are more likely to achieve remission after transition to PP3M.
Keywords: double-blind treatment; long-acting injectable; symptomatic remission.
Conflict of interest statement
Disclosure Nash, Turkoz, Savitz, Mathews, and Kim are employees of Janssen (a Johnson & Johnson company) and hold stock in Johnson & Johnson. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
Factors Associated with Symptom Stabilization that Allow for Successful Transition from Once-Monthly Paliperidone Palmitate to Three-Monthly Paliperidone Palmitate: A Post Hoc Analysis Examined Clinical Characteristics in Chinese Patients with Schizophrenia.CNS Drugs. 2024 Jan;38(1):55-65. doi: 10.1007/s40263-023-01056-x. Epub 2024 Jan 8. CNS Drugs. 2024. PMID: 38190077 Free PMC article. Clinical Trial.
-
Improvement of Negative Symptoms in Schizophrenia with Paliperidone Palmitate 1-Month and 3-Month Long-Acting Injectables: Results from a Phase 3 Non-Inferiority Study.Neuropsychiatr Dis Treat. 2020 Mar 6;16:681-690. doi: 10.2147/NDT.S226296. eCollection 2020. Neuropsychiatr Dis Treat. 2020. PMID: 32184607 Free PMC article.
-
Efficacy and Safety of Paliperidone Palmitate 3-Month Formulation for Patients with Schizophrenia: A Randomized, Multicenter, Double-Blind, Noninferiority Study.Int J Neuropsychopharmacol. 2016 Jul 5;19(7):pyw018. doi: 10.1093/ijnp/pyw018. Print 2016 Jul. Int J Neuropsychopharmacol. 2016. PMID: 26902950 Free PMC article. Clinical Trial.
-
Paliperidone 3-Month Injection for Treatment of Schizophrenia: A Narrative Review.Front Psychiatry. 2021 Sep 21;12:699748. doi: 10.3389/fpsyt.2021.699748. eCollection 2021. Front Psychiatry. 2021. PMID: 34621193 Free PMC article. Review.
-
Dosing and Switching Strategies for Paliperidone Palmitate 3-Month Formulation in Patients with Schizophrenia Based on Population Pharmacokinetic Modeling and Simulation, and Clinical Trial Data.CNS Drugs. 2017 Apr;31(4):273-288. doi: 10.1007/s40263-017-0416-1. CNS Drugs. 2017. PMID: 28258365 Review.
Cited by
-
Paliperidone Palmitate Every Three Months (PP3M) 2-Year Treatment Compliance, Effectiveness and Satisfaction Compared with Paliperidone Palmitate-Monthly (PP1M) in People with Severe Schizophrenia.J Clin Med. 2021 Apr 1;10(7):1408. doi: 10.3390/jcm10071408. J Clin Med. 2021. PMID: 33915786 Free PMC article.
-
Dynamics of Clinical Manifestations and Social Functioning in Schizophrenia: A Non-interventional Observational Study of Paliperidone Palmitat Dosage Forms.Consort Psychiatr. 2024 Dec 13;5(4):16-38. doi: 10.17816/CP15567. eCollection 2024. Consort Psychiatr. 2024. PMID: 39980622 Free PMC article.
-
Aripiprazole Lauroxil Every 2 Months or Paliperidone Palmitate Monthly for Acute Schizophrenia: A Post Hoc Analysis of PANSS Five-Factor Scores in the ALPINE Trial.Neuropsychiatr Dis Treat. 2025 May 14;21:1047-1055. doi: 10.2147/NDT.S510471. eCollection 2025. Neuropsychiatr Dis Treat. 2025. PMID: 40390796 Free PMC article.
-
Long-Term Symptomatic and Functional Remission with Paliperidone Palmitate 6-Monthly Treatment in Schizophrenia: A 3-Year Post-Hoc Analysis.Neuropsychiatr Dis Treat. 2025 Jun 18;21:1203-1214. doi: 10.2147/NDT.S523687. eCollection 2025. Neuropsychiatr Dis Treat. 2025. PMID: 40548350 Free PMC article.
-
Asian Subgroup Analysis of the REMISSIO Study: A Long-Term Efficacy and Safety Study of Paliperidone Palmitate 3-month Formulation in Patients with Stable Schizophrenia in a Naturalistic Clinical Setting.Clin Psychopharmacol Neurosci. 2022 Aug 31;20(3):427-439. doi: 10.9758/cpn.2022.20.3.427. Clin Psychopharmacol Neurosci. 2022. PMID: 35879027 Free PMC article.
References
-
- Lieberman JA, Perkins D, Belger A, et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50(11):884–897. - PubMed
-
- Millier A, Schmidt U, Angermeyer MC, et al. Humanistic burden in schizophrenia: a literature review. J Psychiatr Res. 2014;54:85–93. - PubMed
-
- Jin H, Mosweu I. The societal cost of schizophrenia: a systematic review. Pharmacoeconomics. 2017;35(1):25–42. - PubMed
-
- Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. 2016;77(6):764–771. - PubMed
-
- Hsiao CY, Lee CT, Lu HL, Tsai YF. Living with schizophrenia: health-related quality of life among primary family caregivers. J Clin Nurs. 2017;26(23–24):5151–5159. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

