A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer
- PMID: 30962720
- PMCID: PMC6434912
- DOI: 10.2147/CMAR.S189627
A systematic review of international guidelines and recommendations for the genetic screening, diagnosis, genetic counseling, and treatment of BRCA-mutated breast cancer
Abstract
Purpose: To conduct a systematic review of international guidelines on screening and management of patients with BRCA-mutated breast cancer (BC).
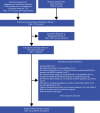
Methods: Major electronic databases (MEDLINE and Embase; N=8) and gray literature sources were searched (January 2007 to February 2018). Latest guideline recommendations on genetic screening, counseling, and BC treatment of BRCA mutation carriers were summarized. Guidelines specific to germline BRCA (gBRCA) mutation were captured where available.
Results: A total of 3,775 records were retrieved and 32 guidelines were included; Europe (n=16), USA (n=11), Canada (n=3), Australia (n=1), and Japan (n=1) were included. Across and within guidelines, genetic counseling was recommended at multiple points in the care pathway, though the format was not always clearly defined. US guidelines emphasized that BRCA mutation testing should occur after specialized genetic counseling; other European guidelines are less prescriptive. BRCA testing eligibility criteria differed, with some guidelines being less restrictive; US National Comprehensive Cancer Network (NCCN) BC guidelines specified that HER2-negative BC patients eligible for single-agent therapy are eligible for gBRCA testing. Fast-track BRCA testing is recommended in the Netherlands if treatment choice will affect survival, but in the UK only as part of clinical trials. More recent European (European School of Oncology-European Society for Medical Oncology 3rd International Consensus Guidelines for Breast Cancer in Young Women 2017, Arbeitsgemeinschaft Gynäkologische Onkologie 2017 in Germany) and US (NCCN) guidelines have updated recommendations regarding gBRCA-targeted poly(ADP-ribose) polymerase (PARP) inhibitor therapy in BC.
Conclusion: Regional and organizational guidelines differ for genetic screening, counseling, and treatment of patients with BRCA-mutated BC. Guideline harmonization would optimize identification and management of these patients.
Keywords: BRCA1; BRCA2; PARP inhibitor; chemotherapy; guidelines; systematic review.
Conflict of interest statement
Disclosure CF, DF, and SdK are employees of Kleijnen Systematic Reviews Ltd. who were paid consultants to Pfizer Inc. in connection with the development of this manuscript. RGWQ is an employee of Pfizer Inc. The authors report no other conflicts of interest with respect to this work.
Figures
References
-
- Greenup R, Buchanan A, Lorizio W, et al. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol. 2013;20(10):3254–3258. - PubMed
-
- National Comprehensive Cancer Network Genetic/familial high-risk assessment: breast and ovarian. Version 1.2018, October 3, 2017. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2017. [Accessed December 15, 2017]. Available from: https://www.nccn.org/
-
- Paluch-Shimon S, Cardoso F, Sessa C, et al. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO clinical practice guidelines for cancer prevention and screening. Ann Oncol. 2016;27(Suppl 5):v103–v110. - PubMed
-
- National Institute for Health and Care Excellence [homepage on the Internet] Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. 2013. [Accessed November 30, 2017]. (NICE clinical guideline CG164). Available from: nice.org.uk/guidance/cg164.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

