Protective Role of Nuclear Factor Erythroid-2-Related Factor 2 against Mechanical Trauma-Induced Apoptosis in a Vaginal Distension-Induced Stress Urinary Incontinence Mouse Model
- PMID: 30962861
- PMCID: PMC6431382
- DOI: 10.1155/2019/2039856
Protective Role of Nuclear Factor Erythroid-2-Related Factor 2 against Mechanical Trauma-Induced Apoptosis in a Vaginal Distension-Induced Stress Urinary Incontinence Mouse Model
Abstract
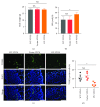
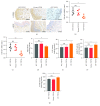
Apoptosis and oxidative damage are involved in the pathogenesis and progression of stress urinary incontinence (SUI). Our previous results indicate that cell apoptosis and oxidative damage increase in a mouse model of mechanical injury-induced SUI and in fibroblasts treated with excessive mechanical strain. Nuclear factor erythroid-2-related factor 2 (Nrf2) is a well-characterized global antioxidant gene inducer that can reduce oxidative damage and apoptosis. Therefore, we predicted that Nrf2 may have a protective role in mechanical trauma-induced SUI. To test this hypothesis, a mouse model of vaginal distension- (VD-) induced SUI was established. Leak point pressure (LPP); levels of apoptosis, apoptosis-related proteins, and peroxidation products; and the activities of antioxidative proteins in the anterior vaginal wall were measured in wild-type (Nfe2l2+/+) C57BL/6 mice and Nrf2-knockout mice (Nfe2l2-/-). The results showed that Nrf2 knockout aggravated VD-induced reduction in LPP, increase in cell apoptosis and peroxidation product levels, decrease in antioxidative protein activities, and alterations in apoptosis-related protein levels in the vaginal walls of mice. To further confirm the role of Nrf2 in mechanical trauma-induced apoptosis and SUI, VD was performed on mice overexpressing Nrf2 via in vivo transfection of LV-Nfe2l2. The results showed that Nrf2 overexpression significantly alleviated VD-induced abnormalities in the anterior vaginal wall. Taken together, our data suggested that Nrf2 is a potential protective factor in mechanical trauma-induced apoptosis in a mouse model of SUI. Antioxidative therapy may be a promising treatment for mechanical trauma-related SUI.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

