Quality standards in respiratory real-life effectiveness research: the REal Life EVidence AssessmeNt Tool (RELEVANT): report from the Respiratory Effectiveness Group-European Academy of Allergy and Clinical Immunology Task Force
- PMID: 30962875
- PMCID: PMC6436229
- DOI: 10.1186/s13601-019-0255-x
Quality standards in respiratory real-life effectiveness research: the REal Life EVidence AssessmeNt Tool (RELEVANT): report from the Respiratory Effectiveness Group-European Academy of Allergy and Clinical Immunology Task Force
Abstract
Introduction: A Task Force was commissioned jointly by the European Academy of Allergy and Clinical Immunology (EAACI) and the Respiratory Effectiveness Group (REG) to develop a quality assessment tool for real-life observational research to identify high-quality real-life asthma studies that could be considered within future guideline development.
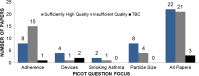
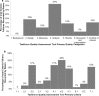
Methods: The resulting REal Life EVidence AssessmeNt Tool (RELEVANT) was achieved through an extensive analysis of existing initiatives in this area. The first version was piloted among 9 raters across 6 articles; the revised, interim, version underwent extensive testing by 22 reviewers from the EAACI membership and REG collaborator group, leading to further revisions and tool finalisation. RELEVANT was validated through an analysis of real-life effectiveness studies identified via systematic review of Medline and Embase databases and relating to topics for which real-life studies may offer valuable evidence complementary to that from randomised controlled trials. The topics were selected through a vote among Task Force members and related to the influence of adherence, smoking, inhaler device and particle size on asthma treatment effectiveness.
Results: Although highlighting a general lack of high-quality real-life effectiveness observational research on these clinically important topics, the analysis provided insights into how identified observational studies might inform asthma guidelines developers and clinicians. Overall, RELEVANT appeared reliable and easy to use by expert reviewers.
Conclusions: Using such quality appraisal tools is mandatory to assess whether specific observational real-life effectiveness studies can be used to inform guideline development and/or decision-making in clinical practice.
Keywords: Asthma; Comparative effectiveness; Database; Observational studies; Quality standards.
Conflict of interest statement
JC discloses prior Respiratory Effectiveness Group funding related to this study, but has no other conflicts of interests associated with this paper. NP reports grants from Gerolymatos, personal fees from Hal Allergy B.V., personal fees from Novartis Pharma AG, personal fees from Menarini, personal fees from Hal Allergy B.V., personal fees from Mylan, outside the submitted work. JK has research funding from the U.S. National Institutes of Health and the U.S. Patient Centered Outcomes Research Institute paid to the University for investigator-initiated research. JB does not serve on advisory boards or have other potential conflicts of interest. GB has, within the last 5 years, received honoraria for lectures from AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Novartis and Teva; he is a member of advisory boards for AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Novartis, Sanofi/Regeneron and Teva. AC and GChave no conflicts of interest to declare in relation to this paper. LB has no perceived COI. MT nor any member of his close family has any shares in pharmaceutical companies. In the last 3 years he has received speaker’s honoraria for speaking at sponsored meetings or satellite symposia at conferences from the following companies marketing respiratory and allergy products: Aerocrine, GSK, Novartis. He has received honoraria for attending advisory panels with; Aerocrine, Boehringer Inglehiem, GSK, MSD, Novartis, Pfizer. He is a recent a member of the BTS SIGN Asthma guideline steering group and the NICE Asthma Diagnosis and Monitoring guideline development group. EVG reports grants and personal fees from ALK ABELLO, grants and personal fees from Bayer, grants and personal fees from BMS, grants and personal fees from GlaxoSmithKline, grants and personal fees from Merck Sharp and Dohme, personal fees from PELyon, outside the submitted work. MB reports grants from GlaxoSmithKline, TEVA, Chiesi, Genentech, outside the submitted work. JQ’sresearch group has received funding from The Health Foundation, MRC, Wellcome Trust, BLF, GSK, Insmed, AZ, Bayer and BI for other projects, none of which relate to this work. Dr Quint has received funds from AZ, GSK, Chiesi, Teva and BI for Advisory board participation or travel. DP has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Mundipharma, Napp, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Mylan, Mundipharma, Napp, Novartis, Pfizer, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Teva Pharmaceuticals, Theravance, UK National Health Service, Zentiva (Sanofi Generics); payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Sanofi Genzyme, Skyepharma, Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma, Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma, Novartis; payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, Teva Pharmaceuticals; funding for patient enrolment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, Zentiva (Sanofi Generics); stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); and is peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, and Health Technology Assessment. NR - Dr. Roche reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from Teva, personal fees from GSK, personal fees from AstraZeneca, personal fees from Chiesi, personal fees from Mundipharma, personal fees from Cipla, grants and personal fees from Pfizer, personal fees from Sanofi, personal fees from Sandoz, personal fees from 3 M, personal fees from Zambon, outside the submitted work. The authors declare that they have no competing interests
Figures

Similar articles
-
The REal Life EVidence AssessmeNt Tool (RELEVANT): development of a novel quality assurance asset to rate observational comparative effectiveness research studies.Clin Transl Allergy. 2019 Mar 27;9:21. doi: 10.1186/s13601-019-0256-9. eCollection 2019. Clin Transl Allergy. 2019. PMID: 30962876 Free PMC article.
-
Real-world research and its importance in respiratory medicine.Breathe (Sheff). 2015 Mar;11(1):26-38. doi: 10.1183/20734735.015414. Breathe (Sheff). 2015. PMID: 26306101 Free PMC article. Review.
-
[Standard technical specifications for methacholine chloride (Methacholine) bronchial challenge test (2023)].Zhonghua Jie He He Hu Xi Za Zhi. 2024 Feb 12;47(2):101-119. doi: 10.3760/cma.j.cn112147-20231019-00247. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38309959 Chinese.
-
Guideline on allergen-specific immunotherapy in IgE-mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto- Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV-HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD).Allergo J Int. 2014;23(8):282-319. doi: 10.1007/s40629-014-0032-2. Allergo J Int. 2014. PMID: 26120539 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
Tactical Considerations for Designing Real-World Studies: Fit-for-Purpose Designs That Bridge Research and Practice.Pragmat Obs Res. 2023 Sep 25;14:101-110. doi: 10.2147/POR.S396024. eCollection 2023. Pragmat Obs Res. 2023. PMID: 37786592 Free PMC article. Review.
-
Perspectives on decisions for treatment and care in severe asthma.World Allergy Organ J. 2021 Jan 16;14(1):100500. doi: 10.1016/j.waojou.2020.100500. eCollection 2021 Jan. World Allergy Organ J. 2021. PMID: 33537114 Free PMC article. Review.
-
Adequacy of Therapy for People with Both COPD and Heart Failure in the UK: Historical Cohort Study.Pragmat Obs Res. 2020 Jun 2;11:55-66. doi: 10.2147/POR.S250451. eCollection 2020. Pragmat Obs Res. 2020. PMID: 32581622 Free PMC article.
-
Real-Life Effectiveness of Subcutaneous Immune Therapy with Carbamylated Monomeric Allergoids on Mite, Grass, and Pellitory Respiratory Allergy: A Retrospective Study.J Clin Med. 2022 Dec 12;11(24):7384. doi: 10.3390/jcm11247384. J Clin Med. 2022. PMID: 36556000 Free PMC article.
-
Allergen immunotherapy for respiratory allergy: Quality appraisal of observational comparative effectiveness studies using the REal Life Evidence AssessmeNt Tool. An EAACI methodology committee analysis.Clin Transl Allergy. 2021 Jun 14;11(4):e12033. doi: 10.1002/clt2.12033. eCollection 2021 Jun. Clin Transl Allergy. 2021. PMID: 34141180 Free PMC article.
References
-
- Roche N, Reddel HK, Agusti A, Bateman ED, Krishnan JA, Martin RJ, et al. Integrating real-life studies in the global therapeutic research framework. Lancet Respir Med. 2013;1(10):e29–e30. - PubMed
-
- Price DB, Swern A, Tozzi CA, Philip G, Polos P. Effect of montelukast on lung function in asthma patients with allergic rhinitis: analysis from the COMPACT trial. Allergy. 2006;61(6):737–742. - PubMed
-
- Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. - PubMed

